Abstract
Benign parotid tumor is one of the most common neoplasms in head and neck region. Its therapeutic methods have been debatable topics over the past 100 years. Recently, some surgeons suggest that extracapsular dissection (ECD) instead of superficial parotidectomy (SP) for treatment of benign parotid tumor. This study aimed to compare ECD with SP in the treatment of benign parotid tumors by a meta-analysis.
We searched Cochrane Library, PubMed, Embase, Ovid, and Web of Science databases on February 14, 2015 for studies that assessed clinical outcomes of SP and ECD as surgical techniques for the management of benign parotid tumors. Outcome data were evaluated by pooled risk ratio (RR) and corresponding 95% confidence interval (CI).
After serious scrutiny, a total of 14 cohort studies with 3194 patients were included in this meta-analysis. The pooled RR revealed that there were no significant difference in tumor recurrence rate between ECD and SP (fixed-effect model: RR = 0.71, 95% CI = 0.40–1.27, P = 0.249; random-effect model: RR = 0.67, 95% CI = 0.38–1.23, P = 0.197). However, there were significantly lower incidences of transient facial nerve dysfunction (FND), permanent FND, and Frey's syndrome in patients of ECD group compared with SP group.
ECD might be a good choice in treatment of the benign parotid tumor that were mobile, small, located in superficial lobe and without adhesion to facial nerve; ECD should be performed by the experienced surgeons with ability of dissection facial nerve, who should perform SP if tumor is found adhere to facial nerve during an operation; and a multicenter randomized control trial study is necessary to decide the optimal treatment of benign parotid tumor.
INTRODUCTION
Salivary gland tumors account for 3% to 10% of all head and neck neoplasms.1–5 About 80% of them originate in the parotid gland, where about 80% of them are benign.6 The guidelines of surgical treatment for benign parotid tumor have been changed on the course of time.
Before the 1940s, the simple surgical technique, enucleation, was a widely used operation because of shortages of understanding of parotid gland and facial nerve anatomy. Around this time, however, some researchers reported the high recurrence rates of enucleation7–9 and a new surgical technique, parotidectomy, was reported.10–12 After its wide use, the recurrence rates for benign parotid tumor decreased dramatically. Therefore, from that time the parotidectomy and/or superficial parotidectomy (SP) became the golden standard treatment of parotid tumors at most medical centers. However, the complications, such as the facial nerve paralysis, Frey's syndrome (FS), and cosmetic deformities, did arise because of the wide use of parotidectomy and SP. As these postoperative complications must not be ignored, several improvements to this surgical technique have been reported over the past several decades, including extracapsular dissection (ECD).
SP is a technique with the whole removal of superficial lobe, dissection and preservation of branches, and main trunk of facial nerve, and also with a total removal of the parotid neoplasm.13,14
ECD is a technique that involves a total excision of the benign parotid tumor surrounded by healthy parotid gland tissue without planed dissection of the main trunk of facial nerve.3,15–18 ECD should be differentiated from enucleation. The latter is a technique which removes the tumor directly at the tumor capsule without any surrounding normal tissue.17 The main difference between ECD and other types of parotidectomy is that it does not expose the facial nerve trunk when it removes the benign parotid tumor.16,17
With the development of surgical techniques, the enucleation has been abandoned in recent year, and total parotidectomy has only been used in malignant parotid tumor or huge benign parotid tumors. However, as for ECD and SP, which one is the more ideal therapeutic method of benign parotid tumors is one of the most debatable topics in head and neck region. Supporters of SP base their evidence on an assumed higher recurrence rate in patients undergoing ECD,19,20 and those who support ECD declare that patients with benign parotid gland have better clinical outcomes and similar recurrence rates undergoing ECD compared to SP.21–26 Even 3 previous meta-analyses had been reported (data were traced back to 2011), their main results were still conflicting.27–29 Owing to existence of debates, many investigations pointing to this topic have emerged in the last 4 years16,24,30–32 and might provide a more comprehensive and reasonable conclusion. Based on this condition mentioned above, we performed a systematic review and meta-analysis to further investigate the advantages and disadvantages of ECD and SP.
MATERIALS AND METHODS
Search Strategy
We searched Cochrane Library, PubMed, Embase, Ovid, and Web of Science on February 14, 2015 for papers in English that met the following search strategies: gland, parotid OR glands, parotid OR parotid glands; and neoplasm OR tumors OR tumor OR neoplasia OR benign neoplasms OR neoplasms, benign OR benign neoplasm OR neoplasm, benign; and ECD OR dissections OR SP OR ECD. Retrieved articles were screened by 2 investigators according to the article type, title and abstract. And irrelevant articles were excluded. References in the related articles were also screened to find missed papers by literature retrieval. Then, all relevant articles were estimated by inclusion/exclusion criteria, as performed below.
Inclusion/Exclusion Criteria
Inclusion criteria: studies that compared SP to ECD concerning the recurrences or complications in treatment of benign parotid tumors; the descriptions of surgical procedures of SP and ECD were consistent with the descriptions of the introduction part; the details of surgical procedures were available from the original articles; article types were case–control study, cohort study, or randomized controlled trial (RCT) study; papers were published in English as full paper; risk ratios (RRs) for estimating recurrence rate or/and incidence of complications were provided or were extractable from the original articles to estimate the associations between SP and ECD; and all included patients with benign parotid tumors.
Exclusion criteria: meta-analysis, review, letter, meeting abstract, case series, case reports, editorial, and non-English papers; papers without sufficient information on assessment of association between SP and ECD; when multiple articles were reported by the same group, those shared datasets were excluded except for the articles with the most comprehensive datasets; and the descriptions of surgical procedures were ambiguous.
Data Extraction
The relevant information of all the included studies was independently extracted by 2 authors (SX and X-FS). Controversies between 2 investigators were solved by the other authors or discussion. The following information were extracted from each included study: publication year, first author, study design, number of subjects, outcomes of patients, surgical selection criteria, follow-up time, and other relevant data.
Quality Assessment
Quality assessment of the eligible studies was performed by 2 authors dependently according to Centre for Evidence Based Medicine (CEBM): Level 1, systematic reviews of RCTs or systematic review of inception cohort studies; Level 2, inception cohort studies or randomized trial or observational study with dramatic effect; Level 3a, systematic reviews of nonrandomized controlled cohort studies; Level 3b, nonrandomized controlled cohort studies or cohort study or control arm of randomized trial; Level 4a, a systematic review of case series, poor quality cohort, poor case–control studies; Level 4b, case series, poor quality cohort, poor case–control study, and historically controlled studies; Level 5, mechanism-based reasoning.33
Statistical Analysis
Data analyses were performed with STATA 11.0 software (Stata Co., College Station, TX). The RR with its corresponding 95% confidence interval (CI) was used to analyze the association between SP and ECD. The inconsistency index I2 was used to estimate the variation caused by heterogeneity.34 When P > 0.10 and I2 < 25%, which assumes that inter-study heterogeneity was not obvious. Both the fixed-effect model (FEM) and random-effect model (REM) were performed to estimate the association between ECD and SP. Potential publication bias was detected by Begger's test and Egger's linear regression.35 Sensitivity analysis was used to identify the underlying effect of the individual studies on pooled RR. As a meta-analysis study, ethical approval of this study is not required. This study was reported following the PRISMA guidelines.
RESULTS
Literature Retrieval and Characteristics of Included Studies
A total of 2694 papers were retrieved by literature retrieval. After cutting duplications, 1854 articles were left, of which 1742 papers were dropped out as being unconformity with our topics. A further 5 potentially relevant articles were gained by screening references of the remaining literatures. After more detailed estimations of the 117 papers, 14 met the inclusion/exclusion criteria and were included in this meta-analysis to evaluate the associations between SP and ECD.3,16,22–24,30–32,36–41 The literature retrieval process was shown in Figure 1.
FIGURE 1.
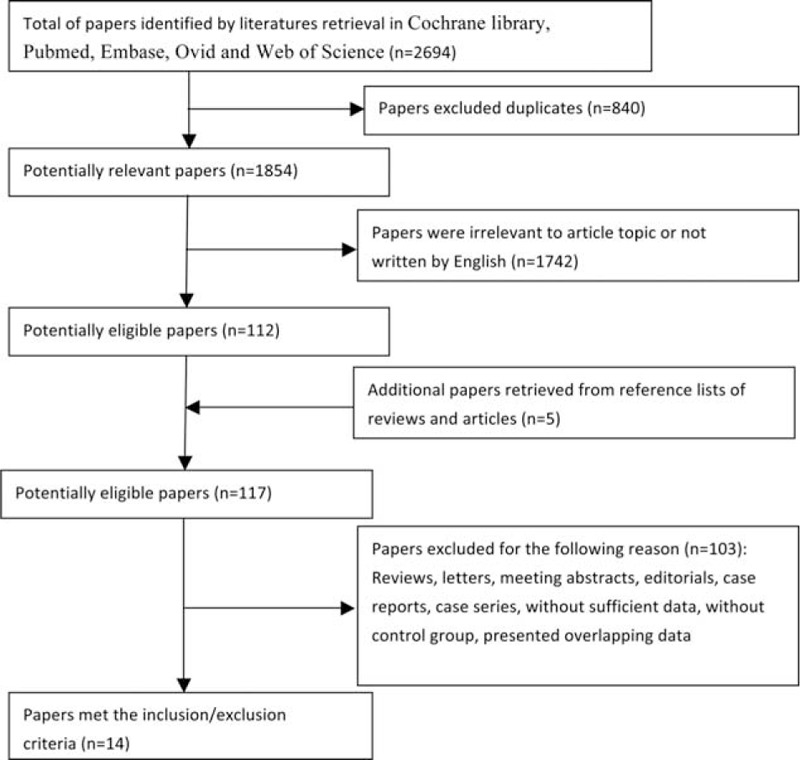
Flow diagram of literature retrieval in this study.
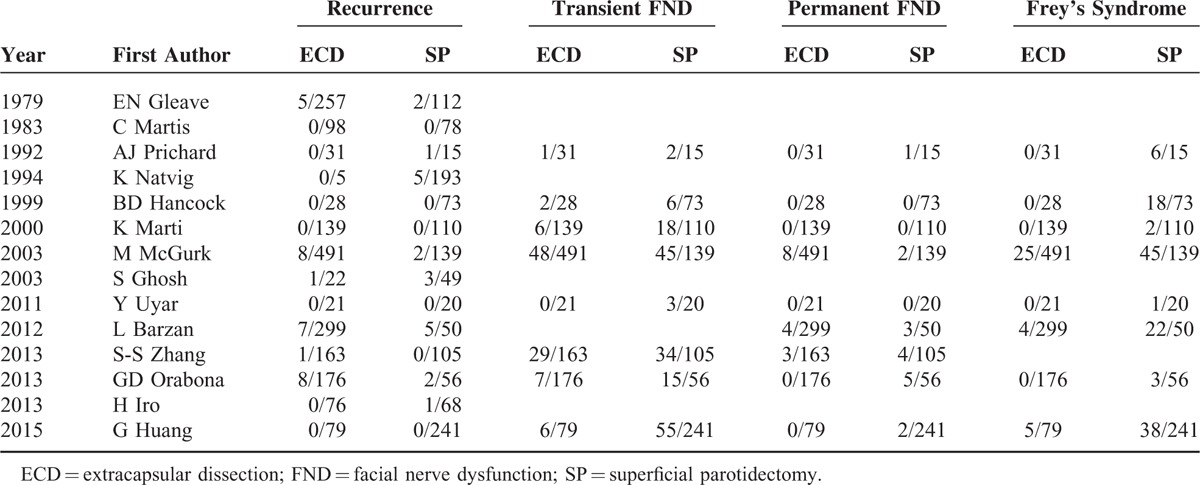
The crucial information extracted from each included study was presented in Table 1, including the publication year, first author, country, number of patients, outcomes of patients, surgical selection criteria, and follow-up and other relevant data. Of these 14 studies, 3194 patients (ECD group: 1885 patients; SP group: 1309 patients) were included to analyze the relevance of SP and ECD. And the publication year of eligible studies ranged from 1979 to 2015. As for the types of included articles, there were 13 retrospective cohort studies, 1 prospective cohort study and none of RCT study. Among these included studies, the majority of them were reported from Europe, and the rest were from Asia. The parameters of clinical outcome included recurrence, transient facial nerve dysfunction (transient FND), permanent FND, and FS. All the detailed data were shown in Tables 1 and 2.
TABLE 1.
Characteristics of Included Cohort Studies
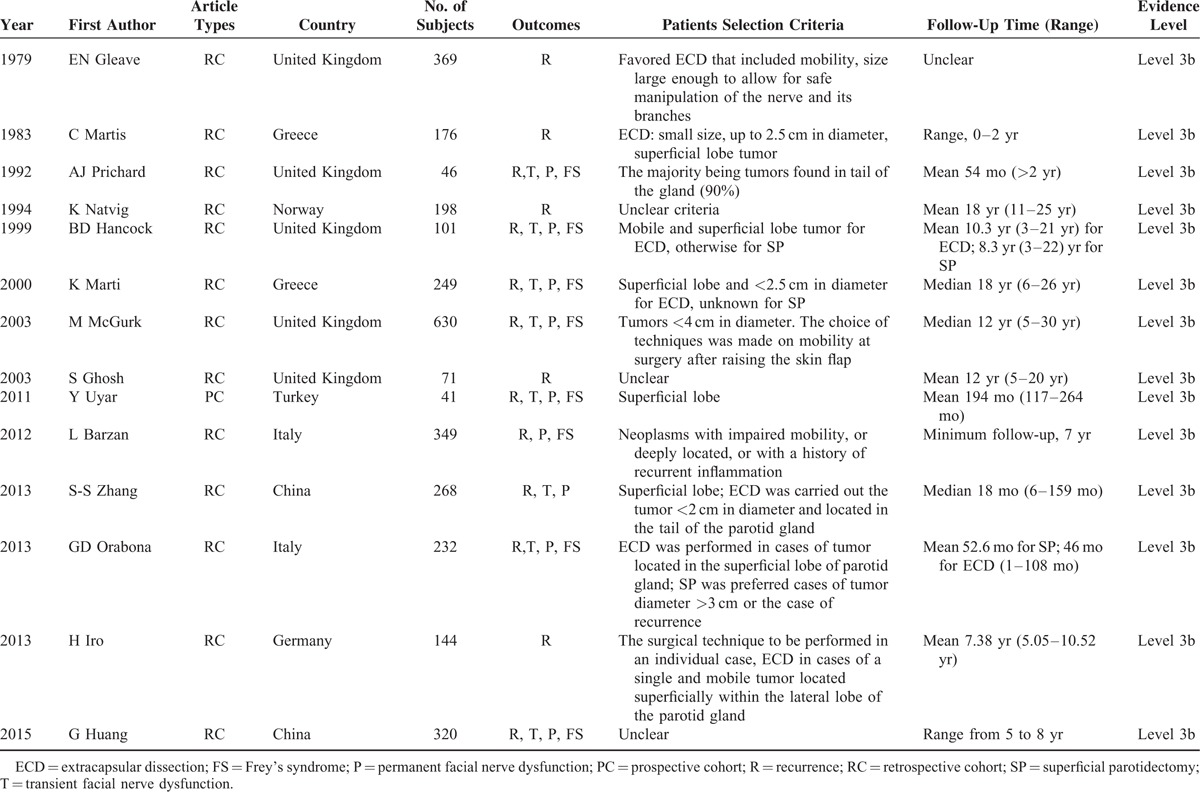
TABLE 2.
Data Summary From Included Studies
The Results of Quality Assessment
All included studies were observational study (cohort study) and the results of quality assessment were shown in Table 1. Because every included study was supported by the Levels 3b of evidence, some certainties in outcomes of systematic review offered a conditional reliability (the levels of evidence: Level 3a).
Recurrence Rates of ECD Versus SP
The results of meta-analysis with regard to recurrence rate showed that there were no obvious heterogeneities among included studies (I2 = 0.0%, P(Q-test) = 0.541). Both the REM and FEM were performed to pool the RRs and 95% CIs. The overall RRs and the corresponding 95% CIs were 0.67 (0.36–1.23) and 0.71 (0.40–1.27), respectively. (Table 3; Figures 2A and 3A), indicating that ECD leads to a similar recurrence rate with SP.
TABLE 3.
Results of ECD Versus SP in This Meta-Analysis
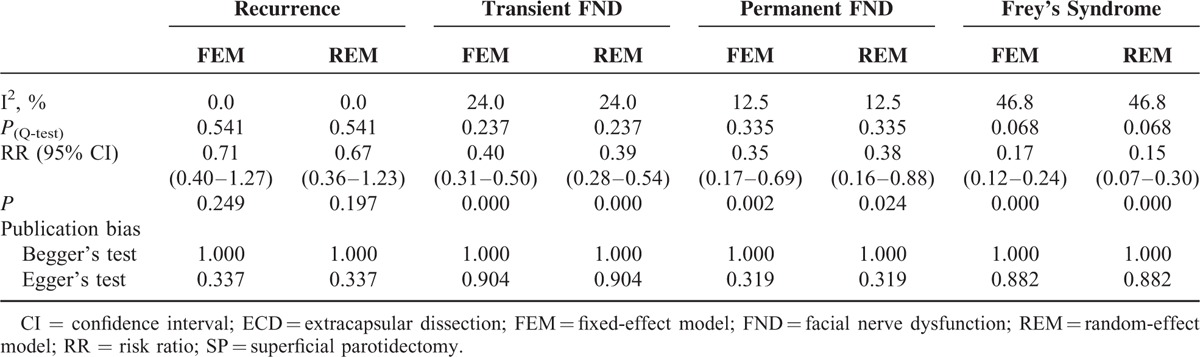
FIGURE 2.
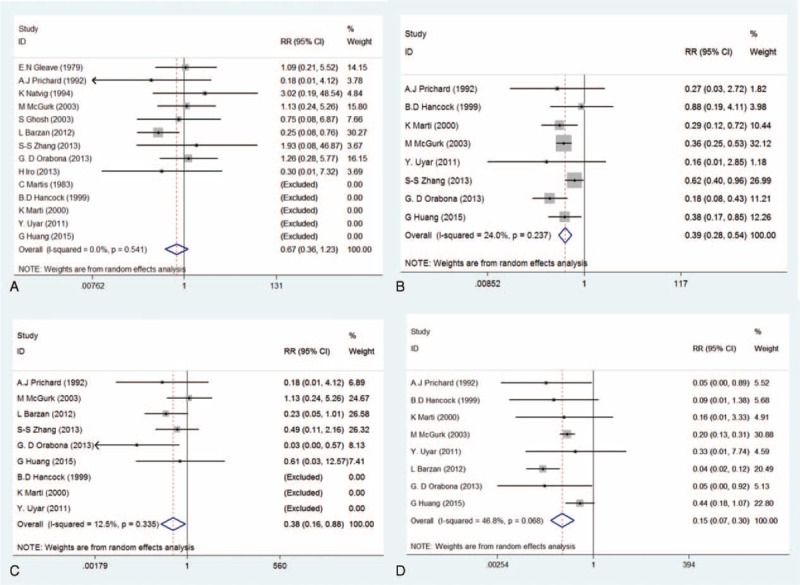
Forest plots evaluating clinical outcomes comparing ECD to SP—random-effect model (A: recurrence; B: transient facial nerve dysfunction; C: permanent facial nerve dysfunction; D: Frey's syndrome). ECD = extracapsular dissection; SP = superficial parotidectomy.
FIGURE 3.
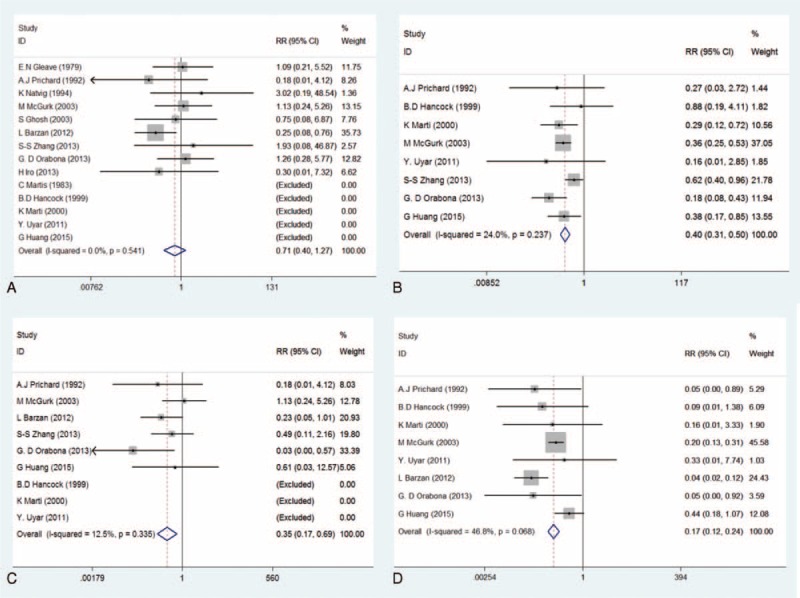
Forest plots evaluating clinical outcomes comparing ECD to SP—fixed-effect model (A: recurrence; B: transient facial nerve dysfunction; C: permanent facial nerve dysfunction; D: Frey's syndrome). ECD = extracapsular dissection; SP = superficial parotidectomy.
Incidence of Transient FND of ECD Versus SP
The result of heterogeneity estimation suggested that the similar homogeneity cross all included studies (I2 = 24.0%, P(Q-test) = 0.237). Both the REM and FEM were used to combined RRs and 95% CIs. The meta-analysis results (RRREM = 0.39, 95% CIREM = 0.28–0.54, P = 0.000; RRFEM = 0.40, 95% CIFEM = 0.31–0.50, P = 0.000) indicated that transient FND has a lower incidence rate with ECD compared with SP (Table 3, Figures 2B and 3B).
Incidence of Permanent FND of ECD Versus SP
Even if fewer heterogeneities existed in these eligible studies (I2 = 12.5%, P(Q-test) = 0.335), both the REM and FEM were carried out to further estimate the incidence of permanent FND. This meta-analysis results (RRREM = 0.38, 95% CIREM = 0.16–0.88, P = 0.024; RRFEM = 0.35, 95% CIFEM = 0.17–0.69, P = 0.002) demonstrated that the incidence of permanent FND was lower in patients with ECD compared with SP (Table 3; Figures 2C and 3C).
Incidence of FS of ECD Versus SP
Owing to moderate heterogeneities in these included studies (I2 = 46.8%, P(Q-test) = 0.068), both the REM and FEM were performed. The results (RRREM = 0.15, 95% CIREM = 0.07–0.30, P = 0.000; RRFEM = 0.17, 95% CIFEM = 0.12–0.24, P = 0.000) suggested that incidence of FS after ECD was significantly lower than that of SP (Table 3; Figures 2D and 3D).
Publication Bias and Sensitivity Analysis
Begger's and Egger's tests were calculated to evaluate the possible publication bias. Begger's and Egger's tests demonstrate that no significant publication bias lies in these meta-analyses (the data were shown in Table 3; Figure 4). The sensitivity analyses produced variations only between the lower CIs limits and the upper CIs limits, suggesting that the data from our meta-analyses are robust and credible (Figure 5).
FIGURE 4.
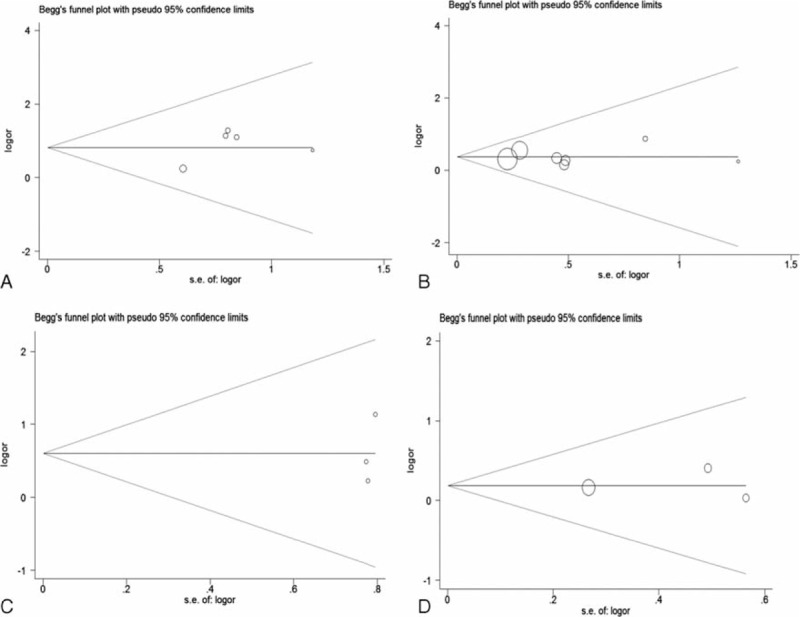
Funnel plots evaluating possible publication bias for clinical outcomes comparing ECD to SP (A: recurrence; B: transient facial nerve dysfunction; C: permanent facial nerve dysfunction; D: Frey's syndrome). ECD = extracapsular dissection; SP = superficial parotidectomy.
FIGURE 5.
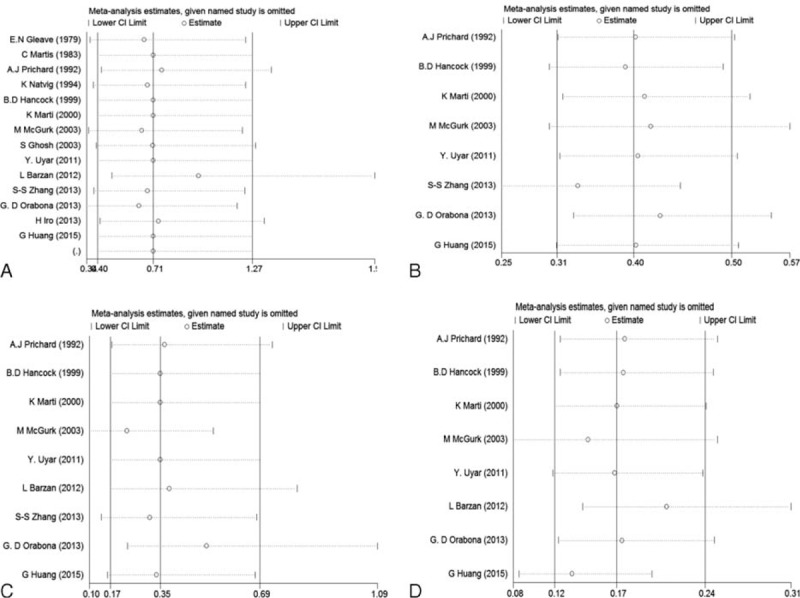
Sensitivity analyses of clinical outcomes comparing ECD to SP (A: recurrence; B: transient facial nerve dysfunction; C: permanent facial nerve dysfunction; D: Frey's syndrome). ECD = extracapsular dissection; SP = superficial parotidectomy.
DISCUSSION
All surgical techniques have moved toward less invasive procedures and reduction in surgical complications, including parotid tumor surgery.42 Owing to abundant complications, the golden standard, SP, treatment of benign parotid tumor has been questioned for many years. How to reach a good balance between tumor recurrence rates and FND has been debatable for several decades. Although lots of published articles or reviews supported the ECD or partial SP instead of SP,17,42,43 dissenters also offered their clinical evidence.19 The debate continues from the birth of ECD to now, even recent systematic reviews, the main conclusions are still conflicting. Foresta et al29 claimed that the recurrence rate is higher in patients undergoing SP compared with ECD; however, Albergotti et al28 declared that ECD has a similar recurrence rate as SP with few surgical complications. To explore the truth of controversy, we scrutinized these meta-analyses and found that they performed different inclusion/exclusion criteria and included different articles, which might produce their conflicting conclusions.
According to the Cochrane handbook, meta-analysis and systematic reviews are considered to be the best available evidence if all good trails are included. However, the best available evidence might not be equal to sufficient evidence.44,45 What is more, lower quality of evidence may lead surgeons to make wrong decisions and kill thousands of lives.46 Thus, it is necessary to recognize the quality of evidence for all clinicians. With the publication of new evidence, it is necessary to reach more reasonable and comprehensive conclusions by using higher levels of evidence. Thus, next we reported a new meta-analysis with better evidence and more serious quality evaluation, including 14 cohort studies with 3194 patients.
In the process of our literature retrieval, we found that the nomenclature and classification system of parotid surgery were disordered among the different authors, even the same surgical procedures also might be defined as different names. Meanwhile, the same surgical names sometimes might be described as different surgical procedures. This meant that the results of some literatures might confuse one thing with another. For example, some authors mistook enucleation for ECD, which might report high recurrence rates and result in false positive. In order to estimate these surgical techniques with unified standards, our inclusion/exclusion criteria were based on their surgical procedures along with their descriptions of materials and methods. After serious scrutiny, 14 cohort studies were included to analyze this topic. In our meta-analysis, the results showed that ECD leads to a similar recurrence rate and fewer surgical complications compared with SP. Besides, the assessment of evidence quality showed our results were supported by a systematic review of cohort studies (Level 3a in evidence grade), which could be considered as conditionally reliable.
However, several shortages should not be ignored. First, the selection bias of surgical patients. The ECD were performed in most of cases of mobile tumor located in the superficial lobe of parotid, and the diameters are <4 cm,22 even 2.5 cm or 2.0 cm.32,39 However, the SP was carried out in most of cases with larger, immobile tumor. The tumor sizes are closely related to tumor recurrence and clinical outcomes. Besides, the bigger tumors might be adhesion to facial nerve trunk and its branches, which might result in facial nerve damage during surgery. Thus, the selection bias reduced the application areas of our results. Second, all included studies were retrospective cohort study, and no prospective RCT was reported. The inner limitation of article type might reduce the credibility and robustness of results. Third, different patients have different genotype and genotype difference may influence the tumor susceptibility, such as Mcl-1, Survivin, rs1447295 and rs4430796 polymorphisms, which might influence the tumor prognosis.47–50 Some surgeons lacking ability to dissect facial nerve might also play a significant role on the clinical outcomes. During the surgical procedures of ECD, SP should be performed if surgeons found that tumor adhere to facial nerve. To benefit patients, all surgeons performing ECD must be experienced and capable to perform multiple variation of the parotid operation. Fourth, owing to long clinical course of parotid tumor, the recurrence time of benign parotid tumor is more than 5 years. The follow-up times of several studies included in our meta-analysis are <7 years, even 2 studies32,39 <5 years, which might produce false-negative data for the recurrence rate. Thus, it is important to interpret the conclusions with caution.
The goal of evidence-based medicine is to find the best evidence to guide clinical decisions. Good decision needs sufficient and good evidence. Based on these conditions mentioned above, we supported rational recommendation with restricted conditions: ECD might be a good choice in treatment of the benign parotid tumor that were mobile, small, located in superficial lobe and without adhesion to facial nerve; ECD should be performed by the experienced surgeons with ability of dissection facial nerve, who should perform SP if tumor is found adhere to facial nerve during an operation; and a multicenter RCT study is necessary to decide the optimal treatment of benign parotid tumor.
Footnotes
Abbreviations: CEBM = Centre for Evidence-Based Medicine, CI = confidence interval, ECD = extracapsular dissection, FEM = fixed-effect model, FND = facial nerve dysfunction, FS = Frey's syndrome, REM = random-effect model, RR = risk ratio, SP = superficial parotidectomy.
This work was supported in part by the grant from the National Natural Science Foundation of China (NSFC 81100762, 81371162, and 30973336). The sponsors had no role in study design, data collection and analysis, the preparation of the manuscript, decision to publish or submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Leverstein H, van der Wal JE, Tiwari RM, et al. Surgical management of 246 previously untreated pleomorphic adenomas of the parotid gland. Br J Surg 1997; 84:399–403. [PubMed] [Google Scholar]
- 2.Iizuka K, Ishikawa K. Surgical techniques for benign parotid tumors: segmental resection vs extracapsular lumpectomy. Acta Otolaryngol Suppl 1998; 537:75–81. [DOI] [PubMed] [Google Scholar]
- 3.Gleave EN, Whittaker JS, Nicholson A. Salivary tumours—experience over thirty years. Clin Otolaryngol Allied Sci 1979; 4:247–257. [DOI] [PubMed] [Google Scholar]
- 4.Smith SL, Komisar A. Limited parotidectomy: the role of extracapsular dissection in parotid gland neoplasms. Laryngoscope 2007; 117:1163–1167. [DOI] [PubMed] [Google Scholar]
- 5.Witt RL. Minimally invasive surgery for parotid pleomorphic adenoma. Ear Nose Throat 2005; 84:308.310–311. [PubMed] [Google Scholar]
- 6.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2807 patients. Head Neck Surg 1986; 8:177–184. [DOI] [PubMed] [Google Scholar]
- 7.McEvedy PG. Diseases of the salivary glands. Clin J 1934; 63:334–338. [Google Scholar]
- 8.McFarland J. Three hundred mixed tumors of the salivary glands, of which sixty-nine recurred. Surg Gynecol Obstet 1936; 63:457–468. [Google Scholar]
- 9.Rawson AJ, Howard JM, Royster HP, et al. Tumors of the salivary glands: a clinicopathological study of 160 cases. Cancer 1950; 3:445–458. [DOI] [PubMed] [Google Scholar]
- 10.Bailey H. Parotidectomy: indications and results. BMJ 1947; 1:404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey H. The surgical anatomy of the parotid gland. BMJ 1948; 2:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janes RM. The treatment of tumours of the salivary gland by radical excision. Can Med Assoc J 1940; 43:554–559. [PMC free article] [PubMed] [Google Scholar]
- 13.Beahrs OH. The surgical anatomy and technique of parotidectomy. Surg Clin North Am 1977; 57:477–493. [DOI] [PubMed] [Google Scholar]
- 14.Olsen KD. Superficial parotidectomy. Oper Techn Gen Surg 2004; 6:102–114. [Google Scholar]
- 15.Klintworth N, Zenk J, Koch M, et al. Postoperative complications after extracapsular dissection of benign parotid lesions with particular reference to facial nerve function. Laryngoscope 2010; 120:484–490. [DOI] [PubMed] [Google Scholar]
- 16.Iro H, Zenk J, Koch M, et al. Follow-up of parotid pleomorphic adenomas treated by extracapsular dissection. Head Neck 2013; 35:788–793. [DOI] [PubMed] [Google Scholar]
- 17.Iro H, Zenk J. Role of extracapsular dissection in surgical management of benign parotid tumors. JAMA Otolaryngol Head Neck Surg 2014; 140:768–769. [DOI] [PubMed] [Google Scholar]
- 18.McGurk M, Renehan A, Gleave EN, et al. Clinical significance of the tumour capsule in the treatment of parotid pleomorphic adenomas. Br J Surg 1996; 83:1747–1749. [DOI] [PubMed] [Google Scholar]
- 19.Piekarski J, Nejc D, Szymczak W, et al. Results of extracapsular dissection of pleomorphic adenoma of parotid gland. J Oral Maxillofac Surg 2004; 62:1198–1202. [DOI] [PubMed] [Google Scholar]
- 20.Witt RL, Rejto L. Pleomorphic adenoma: extracapsular dissection versus partial superficial parotidectomy with facial nerve dissection. Del Med J 2009; 81:119–125. [PubMed] [Google Scholar]
- 21.Roh JL. Extracapsular dissection of benign parotid tumors using a retroauricular hairline incision approach. Am J Surg 2009; 197:e53–e56. [DOI] [PubMed] [Google Scholar]
- 22.McGurk M, Thomas BL, Renehan AG. Extracapsular dissection for clinically benign parotid lumps: reduced morbidity without oncological compromise. Br J Cancer 2003; 89:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock BD. Clinically benign parotid tumours: local dissection as an alternative to superficial parotidectomy in selected cases. Ann R Coll Surg Engl 1999; 81:299–301. [PMC free article] [PubMed] [Google Scholar]
- 24.Orabona GD, Bonavolonta P, Iaconetta G, et al. Surgical management of benign tumors of the parotid gland: extracapsular dissection versus superficial parotidectomy-our experience in 232 cases. J Oral Maxil Surg 2013; 71:410–413. [DOI] [PubMed] [Google Scholar]
- 25.George KS, McGurk M. Extracapsular dissection—minimal resection for benign parotid tumours. Br J Oral Maxillofac Surg 2011; 49:451–454. [DOI] [PubMed] [Google Scholar]
- 26.Riffat F, Mahrous AK, Buchanan MA, et al. Safety of extracapsular dissection in benign superficial parotid lesions. J Maxillofac Oral Surg 2012; 11:407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witt RL. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope 2002; 112:2141–2154. [DOI] [PubMed] [Google Scholar]
- 28.Albergotti WG, Nguyen SA, Zenk J, et al. Extracapsular dissection for benign parotid tumors: a meta-analysis. Laryngoscope 2012; 122:1954–1960. [DOI] [PubMed] [Google Scholar]
- 29.Foresta E, Torroni A, Di Nardo F, et al. Pleomorphic adenoma and benign parotid tumors: extracapsular dissection vs superficial parotidectomy-review of literature and meta-analysis. Oral Surg Oral Med Oral Pathol 2014; 117:663–676. [DOI] [PubMed] [Google Scholar]
- 30.Huang G, Yan G, Wei X, et al. Superficial parotidectomy versus partial superficial parotidectomy in treating benign parotid tumors. Oncol Lett 2015; 9:887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barzan L, Pin M. Extra-capsular dissection in benign parotid tumors. Oral Oncol 2012; 48:977–979. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SS, Ma DQ, Guo CB, et al. Conservation of salivary secretion and facial nerve function in partial superficial parotidectomy. Int J Oral Maxillofac Surg 2013; 42:868–873. [DOI] [PubMed] [Google Scholar]
- 33.Howick J, Chalmers I, Glasziou P, et al. The Oxford levels of evidence 2. Oxford Centre for Evidence-Based Medicine. 2011; http://www.cebm.net/index.aspx?o=5653. [Google Scholar]
- 34.Cochran W. The combination of estimates from different experiments. Biometrics 1954; 10:101–129. [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uyar Y, Caglak F, Keles B, et al. Extracapsular dissection versus superficial parotidectomy in pleomorphic adenomas of the parotid gland. Kulak Burun Bogaz Ihtis Derg 2011; 21:76–79. [DOI] [PubMed] [Google Scholar]
- 37.Prichard AJ, Barton RP, Narula AA. Complications of superficial parotidectomy versus extracapsular lumpectomy in the treatment of benign parotid lesions. J R Coll Surg Edinb 1992; 37:155–158. [PubMed] [Google Scholar]
- 38.Natvig K, Soberg R. Relationship of intraoperative rupture of pleomorphic adenomas to recurrence: an 11–25 year follow-up study. Head Neck 1994; 16:213–217. [DOI] [PubMed] [Google Scholar]
- 39.Martis C. Parotid benign tumors: comments on surgical treatment of 263 cases. Int J Oral Surg 1983; 12:211–220. [DOI] [PubMed] [Google Scholar]
- 40.Marti K, Zografos GC, Martis C. Extracapsular excision of small benign tumors of the parotid gland. J Surg Oncol 2000; 75:208–209. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S, Panarese A, Bull PD, et al. Marginally excised parotid pleomorphic salivary adenomas: risk factors for recurrence and management. A 12.5-year mean follow-up study of histologically marginal excisions. Clin Otolaryngol Allied Sci 2003; 28:262–266. [DOI] [PubMed] [Google Scholar]
- 42.McGurk M. Benign parotid tumours. BMJ 2004; 329:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deschler DG. Extracapsular dissection of benign parotid tumors. JAMA Otolaryngol Head Neck Surg 2014; 140:770–771. [DOI] [PubMed] [Google Scholar]
- 44.Xie S, Xu H, Shan X, et al. Clinicopathological and prognostic significance of Survivin expression in patients with oral squamous cell carcinoma: evidence from a meta-analysis. PLoS ONE 2015; 10: e0116517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie S, Shan XF, Shang K, et al. Relevance of LIG4 gene polymorphisms with cancer susceptibility: evidence from a meta-analysis. Sci Rep 2014; 4:6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu M, Kurosaki T, Suzuki M, et al. Significance of common variants on human chromosome 8q24 in relation to the risk of prostate cancer in native Japanese men. BMC Genet 2009; 10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Suzuki M, Arai T, et al. A replication study examining three common single-nucleotide polymorphisms and the risk of prostate cancer in a Japanese population. Prostate 2011; 71:1023–1032. [DOI] [PubMed] [Google Scholar]
- 49.Heiduschka G, Erovic BM, Pammer J, et al. Mcl-1 expression is up-regulated in malignancies of the parotid gland. Dis Markers 2011; 30:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenner M, Demgensky A, Molls C, et al. Prognostic value of Survivin expression in parotid gland cancer in consideration of different histological subtypes. Eur J Cancer 2011; 47:1013–1020. [DOI] [PubMed] [Google Scholar]


