Supplemental Digital Content is available in the text
Abstract
Splanchnic vein thrombosis (SVT) refers to Budd–Chiari syndrome (BCS) and portal vein system thrombosis (PVST). Current practice guidelines have recommended the routine screening for antiphospholipid antibodies (APAs) in patients with SVT.
A systematic review and meta-analysis of observational studies was performed to explore the association between APAs and SVT.
The PubMed, EMBASE, and ScienceDirect databases were searched for all relevant papers, in which the prevalence of positive APAs or levels of APAs should be compared between BCS or noncirrhotic PVST patients versus healthy controls, or between cirrhotic patients with portal vein thrombosis (PVT) versus those without PVT.
Fourteen studies were eligible. Only 1 study evaluated the role of APAs in BCS patients and found that positive immunoglobulin (Ig) G anticardiolipin antibody (aCL) was more frequently observed in BCS patients than in healthy controls; however, the associations of other APAs with BCS were not evaluated. Positive IgG aCL was more frequently observed in noncirrhotic patients with PVST than in healthy controls; however, other APAs, such as IgM aCL, lupus anticoagulants (LAs), anti-β2-glycoprotein-I antibody (aβ2GPI), and aβ2GPI-oxidized low-density lipoprotein antibody (ox-LDL) were not associated with noncirrhotic PVST. Positive unclassified aCL was more frequently observed in cirrhotic patients with PVT than in those without PVT; however, the association of IgG aCL and IgM aCL with the development of PVT in liver cirrhosis remained inconsistent among studies.
The risk of BCS and noncirrhotic PVST might be increased by positive IgG aCL but not IgM aCL, LA, aβ2GPI, or aβ2GPI ox-LDL. However, the evidence regarding APAs in BCS originated from only 1 study. The association between APAs and PVT in liver cirrhosis was unclear.
INTRODUCTION
Splanchnic vein thrombosis (SVT) consists of Budd–Chiari syndrome (BCS) and portal venous system thrombosis (PVST).1,2 The former is characterized by the hepatic venous outflow obstruction after the exclusion of sinusoidal obstructive syndrome. The latter is further classified as portal vein thrombosis (PVT), mesenteric vein thrombosis, and splenic vein thrombosis. Currently, the practice guideline regarding the vascular disorders of the liver has recommended that several thrombotic risk factors should be routinely screened in SVT patients.3,4 Antiphospholipid syndrome is regarded as one of the widely accepted thrombotic risk factors, which is defined as a classical triad of arterial and/or venous thrombosis, recurrent fetal loss, and thrombocytopenia in the presence of antiphospholipid antibodies (APAs).5,6 APAs primarily include lupus anticoagulant (LA), anticardiolipin antibody (aCL), anti-β2-glycoprotein-I antibody (aβ2GPI), antiprothrombin, antiphosphatidyl serine, and antiphosphatidyl ethanolamine. Previous systematic reviews have confirmed that these antibodies themselves may be strongly related to the development of thrombotic events within the usual sites.7–11 Notably, the highest risks of thrombosis are associated with LA and immunoglobulin (Ig) G aCL/aβ2GPI isotype and with an antibody profile including triple positivity for LA, aCL, and aβ2GPI.12–14 Herein, we performed a systematic review and meta-analysis of observational studies to explore the associations between APAs and SVT.
METHODS
Search Strategy
The PubMed, EMBASE, and ScienceDirect databases were searched for the relevant papers. The search items are listed in the Appendix. The last search was performed on January 7, 2014.
Eligibility Criteria
Eligibility criteria were as follows: the type of papers should be clinical studies but not reviews, comments, or basic studies; the sample size should be ≥10; the participants should be diagnosed with SVT with or without liver cirrhosis; the participants with hepatocellular carcinoma (HCC) should be excluded, because SVT might be attributed to the tumor invasion in HCC; if the case group was BCS or noncirrhotic patients with PVST, the control group should be healthy subjects; if the case group was cirrhotic patients with SVT, the control group should be cirrhotic patients without SVT; the APAs should be detected in both case and control groups; the publication language and form were not limited. If the data were overlapped among 2 or more studies by the same study team, we extracted the data from 1 study with a larger sample size and/or a longer enrollment period.
Data Extraction
The following data were extracted: first author, publication journal, publication year, country, enrollment period, eligibility criteria, total number of cases and controls, age, sex, methods of APA measurement, proportion of positive APAs in case and control groups, cutoff values for positive APAs, and levels of APAs in case and control groups.
Study Quality
The study quality was scored by the Newcastle–Ottawa scale, including selection, comparability, and outcome categories. Based on the Newcastle–Ottawa scale, a study can be awarded a maximum of 9 points. Studies with scores of 5 points or more were considered to be of high quality.
Data Synthesis
Continuous data were evaluated by a mean difference with 95% confidence interval (CI). Then, the mean difference of each study was combined to give a pooled mean difference. Dichotomous data were evaluated by an odds ratio (OR) with 95% CI. Then, the OR of each study was combined to give a pooled OR. A P value of <0.05 was considered statistically significant for the effect size. Data were pooled by using a random-effects model. Heterogeneity between studies was assessed by using the I2 statistic (I2 > 50% was considered as having substantial heterogeneity) and the χ2 test (P < 0.10 was considered to represent significant statistical heterogeneity). All analyses were conducted using the statistical package Review Manager version 5.2 (Copenhagen, The Nordic Cochrane Center, The Cochrane Collaboration, 2011).
RESULTS
Study Selection
Overall, 1700 papers were retrieved via the 3 databases. Among them, 18 studies were eligible. However, 4 studies were further excluded, because the levels of APAs were reported in SVT patients with HCC in 2 studies,15,16 the enrollment period was shorter in 1 study,17 and only the combined data regarding the biological antiphospholipid syndrome (aCLs and LA) were given in 1 study.18 Thus, 14 studies were finally included in the systematic review19–32 (Figure 1). Notably, 5 studies conducted by the same study team were included,20–24 because the APA tests, types of patients, and/or enrollment periods were different among them.
FIGURE 1.
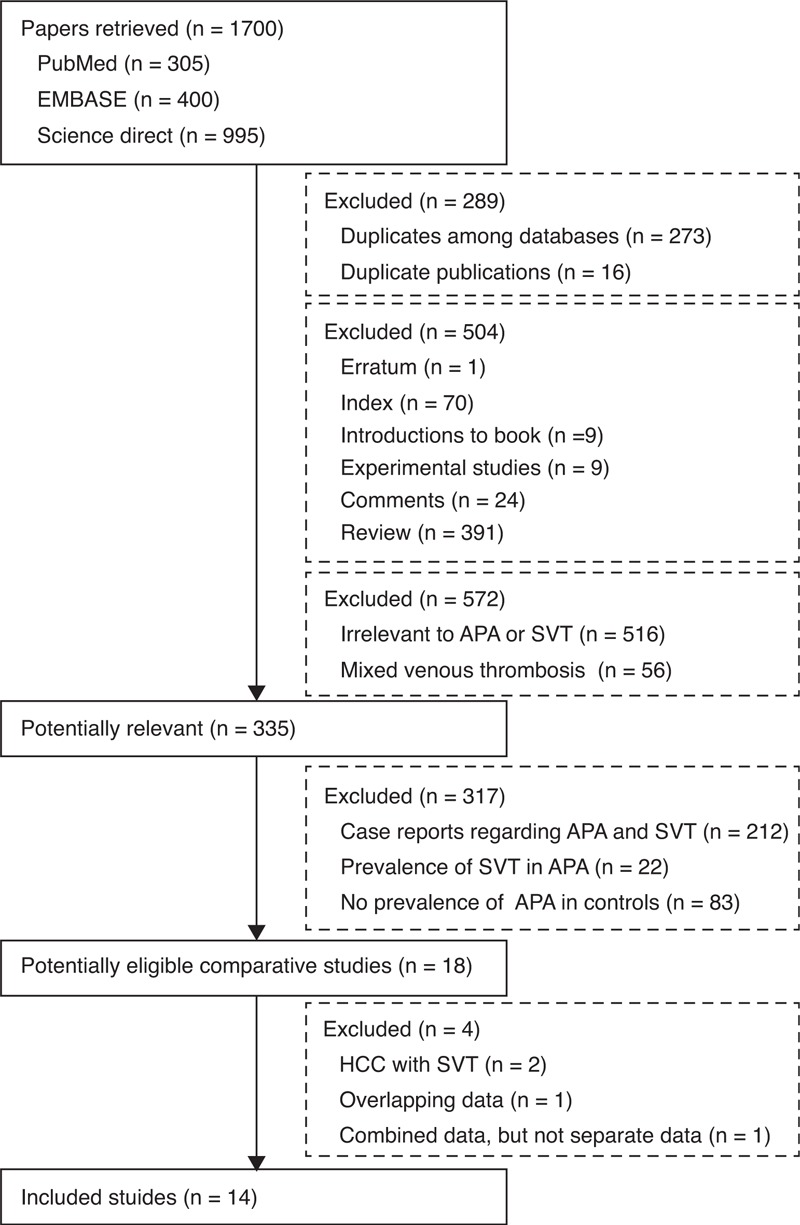
Flowchart of study inclusion. APA = antiphospholipid antibody, HCC = hepatocellular carcinoma, SVT = splanchnic vein thrombosis.
Study Characteristics
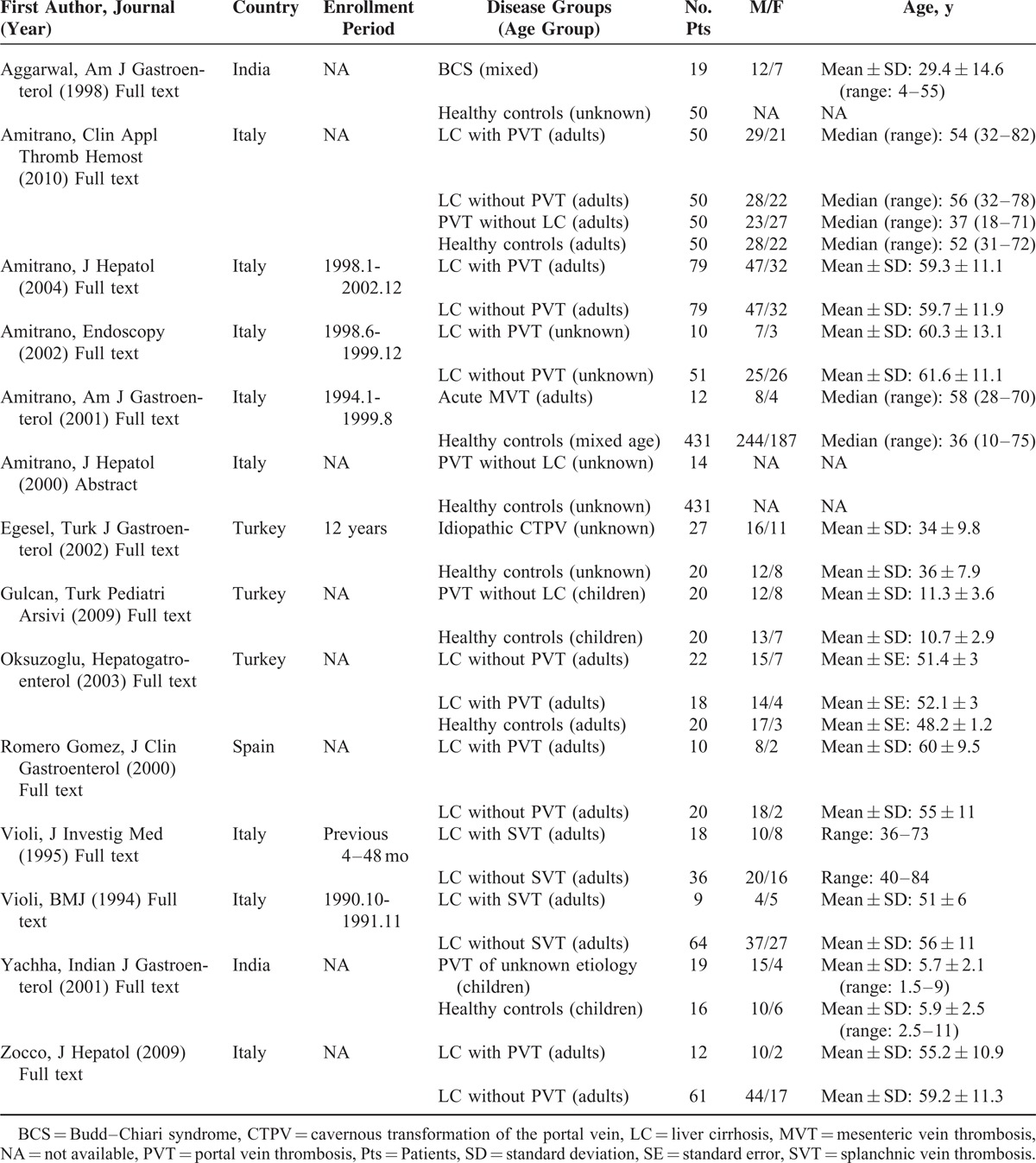
The characteristics of these included studies were summarized in Table 1. All included studies were conducted in Europe and Asia, including Italy (n = 8), Turkey (n = 3), India (n = 2), and Spain (n = 1). One study enrolled BCS patients,19 5 studies enrolled noncirrhotic patients with PVST alone,22,24–26,31 7 studies enrolled cirrhotic patients with PVT alone,21,23,27–30,32 and 1 study enrolled both cirrhotic and noncirrhotic patients with PVT.20 Eligibility criteria and methods of APA measurement were summarized in Supplementary Tables 1 and 2, http://links.lww.com/MD/A196, respectively.
TABLE 1.
Characteristics of Included Studies
Study Quality
Of these included studies, 13 were considered to be of relatively high quality (Supplementary Table 3, http://links.lww.com/MD/A196).
Meta-Analyses
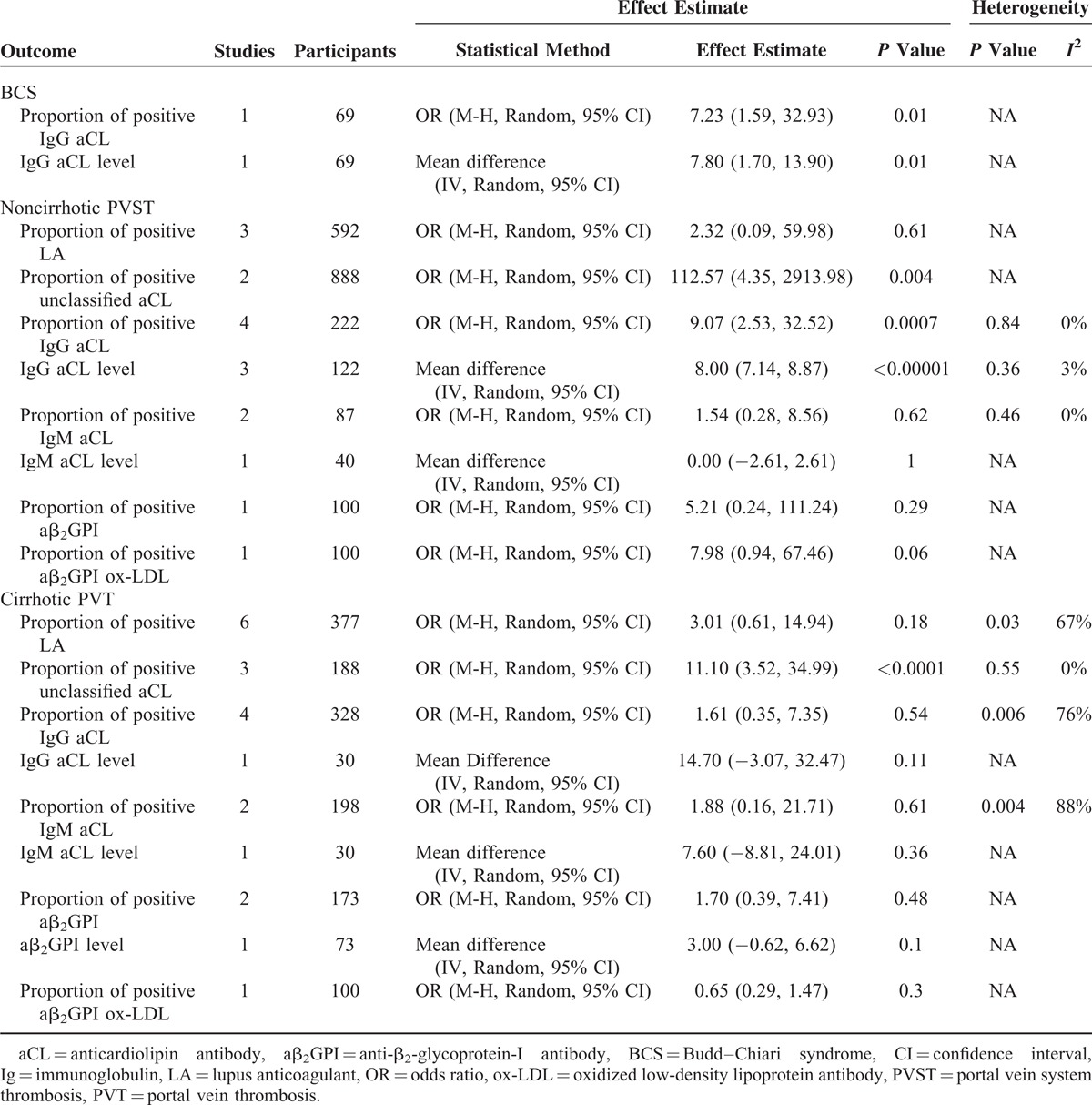
The relevant data from every included study were summarized in Supplementary Tables 4–22, http://links.lww.com/MD/A196. Results of systematic reviews and meta-analyses were summarized in Table 2.
TABLE 2.
Results of Meta-Analyses
Budd–Chiari Syndrome
Immunoglobulin G aCL
BCS patients were investigated in only 1 study, and had a significantly higher proportion of positive IgG aCL or IgG aCL level than healthy controls.19
Noncirrhotic PVST
Lupus Anticoagulant
Meta-analysis of 3 studies demonstrated that the proportion of positive LA was not significantly different between noncirrhotic patients with PVST and healthy controls.20,24,25 Notably, 2 of them showed that the prevalence of positive LA was 0 in either noncirrhotic patients with PVST or healthy controls.20,24
Unclassified aCL
Meta-analysis of 2 studies demonstrated that the proportion of positive unclassified aCL was significantly higher in noncirrhotic patients with PVST than in healthy controls.22,24 Notably, 1 of them showed that the prevalence of positive unclassified aCL was 0 in either noncirrhotic patients with PVST or healthy controls.24
Immunoglobulin G aCL
Meta-analysis of 4 studies demonstrated that the proportion of positive IgG aCL was significantly higher in noncirrhotic patients with PVST than in healthy controls (Figure 2).20,25,26,31 Notably, 1 of them showed that the prevalence of positive IgG aCL was 0 in either noncirrhotic patients with PVST or healthy controls.20 In addition, meta-analysis of 3 studies demonstrated that the IgG aCL level was significantly higher in noncirrhotic patients with PVST than in healthy controls.25,26,31
FIGURE 2.
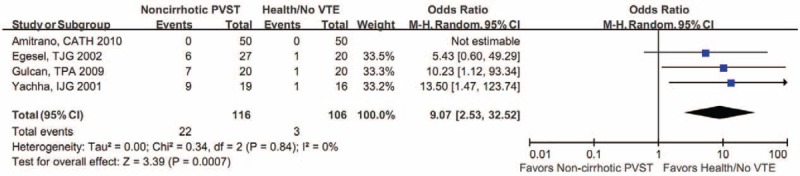
Forest plot comparing the proportion of positive IgG aCL between noncirrhotic patients with PVST and healthy controls without venous thromboembolism. aCL = anticardiolipin antibody, CI = confidence interval, Ig = immunoglobulin, PVST = portal vein system thrombosis, VTE = venous thromboembolism.
Immunoglobulin M aCL
Meta-analysis of 2 studies demonstrated that the proportion of positive IgM aCL was not significantly different between noncirrhotic patients with PVST and healthy controls.25,26 In addition, 1 study demonstrated that the IgG aCL level was not significantly different between the 2 groups.26
Anti-β2-Glycoprotein-I Antibody
Only 1 study demonstrated that the proportion of positive aβ2GPI was not significantly different between noncirrhotic patients with PVST and healthy controls.20
aβ2GPI-Oxidized Low-Density Lipoprotein Antibody
Only 1 study demonstrated that the proportion of positive aβ2GPI-oxidized low-density lipoprotein antibody (ox-LDL) was not significantly different between noncirrhotic patients with PVST and healthy controls.20
Cirrhotic PVT
Lupus Anticoagulant
Meta-analysis of 6 studies demonstrated that the proportion of positive LA was not significantly different between cirrhotic patients with and without PVT (Figure 3).20,23,27,28,30,32 Notably, 2 of them showed that the prevalence of positive LA was 0 in both cirrhotic patients with and without PVT.20,23
FIGURE 3.
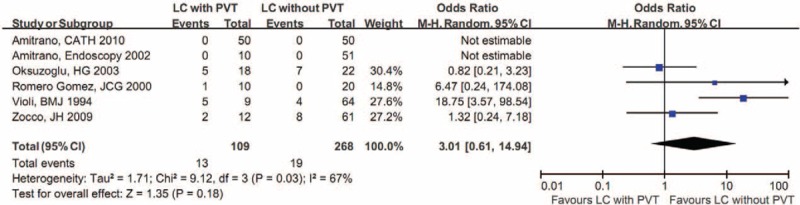
Forest plot comparing the proportion of positive LA between cirrhotic patients with and without PVT. CI = confidence interval, LA = lupus anticoagulant, LC = liver cirrhosis, PVT = portal vein thrombosis.
Unclassified aCL
Meta-analysis of 3 studies demonstrated that the proportion of positive unclassified aCL was significantly higher in cirrhotic patients with PVT than in those without PVT.23,29,30
Immunoglobulin G aCL
Meta-analysis of 4 studies demonstrated that the proportion of positive IgG aCL was not significantly different between cirrhotic patients with and without PVT (Figure 4).20,23,27,28 In addition, the IgG aCL level was expressed as mean with standard deviation in 1 study,28 and as median with interquartile ratio in another study.27 Therefore, a meta-analysis regarding IgG aCL level could not be performed. In details, the former study reported that the IgG aCL level was not significantly different between the 2 groups,28 but the latter study found that IgG aCL level was significantly higher in cirrhotic patients with PVT than in those without PVT (P = 0.014).27
FIGURE 4.
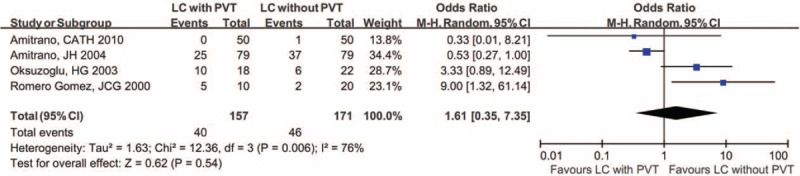
Forest plot comparing the proportion of positive IgG aCL between cirrhotic patients with and without PVT. aCL = anticardiolipin antibody, CI = confidence interval, IgG = immunoglobulin G, LC = liver cirrhosis, PVT = portal vein thrombosis.
Immunoglobulin M aCL
Meta-analysis of 2 studies demonstrated that the proportion of positive IgM aCL was not significantly different between cirrhotic patients with and without PVT.21,27 In addition, the IgM aCL level was expressed as mean with standard deviation in 1 study28 and as median with interquartile ratio in another study.27 Therefore, a meta-analysis regarding IgM aCL level could not be performed. In details, the former study reported that the IgM aCL level was not significantly different between the 2 groups,28 but the latter study found that the IgM aCL level was significantly higher in cirrhotic patients with PVT than in those without PVT (P = 0.001).27
Anti-β2-Glycoprotein-I Antibody
Meta-analysis of 2 studies demonstrated that the proportion of positive aβ2GPI was not significantly different between cirrhotic patients with and without PVT.20,32 Notably, 1 of them demonstrated that the prevalence of positive aβ2GPI was 0 in both cirrhotic patients with and without PVT.20 In addition, 1 study demonstrated that the aβ2GPI level was not significantly different between the 2 groups.32
Anti-β2-Glycoprotein-I Antibody ox-LDL
Only 1 study demonstrated that the proportion of positive aβ2GPI ox-LDL was not significantly different between cirrhotic patients with and without PVT.20
DISCUSSION
Our previous works have systematically evaluated the role of several thrombotic risk factors in the development of SVT, including JAK2 V617F mutation, inherited antithrombin, protein C and protein S deficiencies, factor V Leiden and prothrombin G20210A mutation, methylenetetrahydrofolate reductase C677T mutation, and hyperhomocysteinemia.33–36 The present systematic review has for the first time collected all available evidence regarding the associations between APAs and SVT. The important findings were as follows. First, IgG aCL was positively associated with BCS. Second, unclassified aCL was positively associated with the development of PVST in noncirrhotic patients; and this positive association was attributed to IgG type but not IgM type. Third, LA, aβ2GPI, and aβ2GPI ox-LDL were not associated with the development of PVST in noncirrhotic patients. Fourth, unclassified aCL was positively associated with the development of PVT in cirrhotic patients, but this positive association could not be achieved in the meta-analyses regarding IgG or IgM aCL. Fifth, LA, aβ2GPI, and aβ2GPI ox-LDL were not associated with the development of PVT in cirrhotic patients.
On the basis of an association of IgG aCL with BCS and noncirrhotic PVST, the routine screening for IgG aCL should be recommended. However, the relevant data were very limited in BCS patients, which might influence the reproducibility of our conclusion. Additionally, we would like to emphasize that the significance of other APAs in the pathogenesis of BCS and noncirrhotic PVST should be greatly toned down. Accordingly, the screening tests for LA and IgM aCL might be unnecessary in such patients.
Despite a positive association between unclassified aCL and PVT in liver cirrhosis, we did not establish any positive associations of IgG aCL or IgM aCL with PVT. To explain the unexpected phenomenon, we rechecked the data from every individual study. In the meta-analysis regarding unclassified aCL, all of the 3 included studies demonstrated a higher incidence of positive unclassified aCL in cirrhotic patients with PVT.23,29,30 By comparison, in the meta-analysis regarding IgG aCL, 2 of the 4 included studies demonstrated a higher proportion of positive IgG aCL in cirrhotic patients with PVT,27,28 and another 2 studies with a relatively larger sample size achieved the opposite results.20,21 Furthermore, the cutoffs for positive IgG aCL were close (20 U/mL or 23 GPI units) in the former 2 studies27,28 but very different (10 U/mL or 40 GPI units) in the latter 2 studies.20,21 It should be noted that either an underestimated or overestimated cutoff might result in the reporting bias. In the study with a cutoff of 10 U/mL, 32% of cirrhotic patients with PVT had a positive IgG aCL, and 47% of cirrhotic patients without PVT had a positive IgG aCL.21 By contrast, in the study with a cutoff of 40 GPL units, none of cirrhotic patients with PVT had a positive IgG aCL, and only 2% of cirrhotic patients without PVT had a positive IgG aCL.20 Given the heterogeneous cutoffs among studies, the association needed to be further confirmed in studies with a larger sample size and an appropriate cutoff for positive IgG aCL.
On the other hand, positive APAs could be frequently found in chronic hepatitis virus C infection-related liver diseases without any evidence of venous thrombosis.37–43 Positive APAs were regarded as an epiphenomenon of chronic liver injury,38,42 which might be produced due to the immunologic disturbances induced by hepatitis C virus infection or prolonged tissue damage in systemic organs.43 Biron et al38 also found that the proportion of positive APAs was positively associated with the severity of liver dysfunction. Certainly, we arbitrarily selected liver cirrhosis without PVT as the control group to balance the potential bias caused by the presence of liver diseases.
The major limitation of this study was that evidence concerning BCS patients is restricted to only 1 study, and that a relatively small number of studies concerning PVST were included in every meta-analysis, especially in the meta-analyses regarding aβ2GPI and aβ2GPI ox-LDL. All included studies had a small sample size. In addition, no study investigated the presence of triple-positive APA profiles, which is relevant in the development of the thrombotic risk.12–14 Moreover, we had to acknowledge that that our search strategy was extensive via the 3 major databases. This suggested the necessity of further validation studies in this field.
In conclusion, based on the currently available evidence, IgG aCL was positively associated with the development of BCS and noncirrhotic PVST. However, other APAs might not be considered as the potential thrombotic risk factors for BCS and noncirrhotic PVST. Notably, given that the evidence regarding APAs in BCS originated from only 1 study, the conclusion should be confirmed in more studies. The association between aCL and the development of PVT in liver cirrhosis needed to be further explored.
Appendix
Search #1: (portal vein thrombosis) OR (portal venous thrombosis) OR (portal vein thrombus) OR (portal venous thrombus) OR (portal vein obstruction) OR (portal venous obstruction) OR (portal vein occlusion) OR (portal venous occlusion) OR (thrombotic portal vein) OR (thrombosed portal vein) OR (occluded portal vein) OR (occlusive portal vein) OR (obstructed portal vein) OR (obstructive portal vein) OR (portal cavernoma) OR (cavernous transformation of portal vein) OR (Budd Chiari) OR (hepatic vein thrombosis) OR (hepatic venous thrombosis) OR (hepatic vein obstruction) OR (hepatic venous obstruction) OR (hepatic vein occlusion) OR (hepatic venous occlusion) OR (mesenteric vein thrombosis) OR (mesenteric venous thrombosis) OR (mesenteric vein obstruction) OR (mesenteric venous obstruction) OR (mesenteric vein occlusion) OR (mesenteric venous occlusion) OR (splenic vein thrombosis) OR (splenic venous thrombosis) OR (splenic vein obstruction) OR (splenic venous obstruction) OR (splenic vein occlusion) OR (splenic venous occlusion) OR (splanchnic vein thrombosis) OR (splanchnic venous thrombosis) OR (splanchnic vein obstruction) OR (splanchnic venous obstruction) OR (splanchnic vein occlusion) OR (splanchnic venous occlusion) OR (abdominal vein thrombosis) OR (abdominal venous thrombosis) OR (abdominal vein obstruction) OR (abdominal venous obstruction) OR (abdominal vein occlusion) OR (abdominal venous occlusion) OR (portosplenomesenteric vein thrombosis) OR (portosplenomesenteric venous thrombosis) OR (portosplenomesenteric vein occlusion) OR (portosplenomesenteric venous occlusion) OR (portosplenomesenteric vein obstruction) OR (portosplenomesenteric venous obstruction)
Search #2: (antiphospholipid) OR (anticardiolipin) OR (lupus anticoagulant) OR (anti-β2-glycoprotein I) OR (anti-beta2-glycoprotein I) OR (anti-phosphatidylcholine) OR (anti-phosphatidylethanolamine) OR (anti-phosphatidylinositol) OR (anti-phosphatidylserine) OR (anti-sphingomyeline)
Search #3: #1 AND #2
Footnotes
Abbreviations: aCL = anticardiolipin antibody, APA = antiphospholipid antibody, aβ2GPI = anti-β2-glycoprotein-I antibody, BCS = Budd–Chiari syndrome, CI = confidence interval, HCC = hepatocellular carcinoma, LA = lupus anticoagulant, OR = odds ratio, ox-LDL = oxidized low-density lipoprotein antibody, PVST = portal vein system thrombosis, PVT = portal vein thrombosis, SVT = splanchnic vein thrombosis.
XQ designed the study, performed the literature search and selection, data extraction, quality assessment, and statistical analysis, and drafted the manuscript. CS performed the literature search and selection. MB performed the data extraction and quality assessment. VDS, XG, and DF gave critical comments and revised the manuscript. All authors have made an intellectual contribution to the manuscript and approved the submission.
XG and DF are joint senior authors.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.De Stefano V, Martinelli I. Splanchnic vein thrombosis: clinical presentation, risk factors and treatment. Intern Emerg Med 2010; 5:487–494. [DOI] [PubMed] [Google Scholar]
- 2.Riva N, Donadini MP, Dentali F, et al. Clinical approach to splanchnic vein thrombosis: risk factors and treatment. Thromb Res 2012; 130 suppl 1:S1–S3. [DOI] [PubMed] [Google Scholar]
- 3.DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senzolo M, Riggio O, Primignani M. Vascular disorders of the liver: recommendations from the Italian Association for the Study of the Liver (AISF) ad hoc committee. Dig Liver Dis 2011; 43:503–514. [DOI] [PubMed] [Google Scholar]
- 5.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). JTH 2006; 4:295–306. [DOI] [PubMed] [Google Scholar]
- 6.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763. [DOI] [PubMed] [Google Scholar]
- 7.Reynaud Q, Lega JC, Mismetti P, et al. Risk of venous and arterial thrombosis according to type of antiphospholipid antibodies in adults without systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev 2014; 13:595–608. [DOI] [PubMed] [Google Scholar]
- 8.Sciascia S, Sanna G, Murru V, et al. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost 2014; 111:354–364. [DOI] [PubMed] [Google Scholar]
- 9.Galli M, Luciani D, Bertolini G, et al. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003; 101:1827–1832. [DOI] [PubMed] [Google Scholar]
- 10.Greaves M. Antiphospholipid antibodies and thrombosis. Lancet 1999; 353:1348–1353. [DOI] [PubMed] [Google Scholar]
- 11.Wahl DG, Guillemin F, de Maistre E, et al. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22. [DOI] [PubMed] [Google Scholar]
- 12.Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118:4714–4718. [DOI] [PubMed] [Google Scholar]
- 13.Pengo V, Biasiolo A, Pegoraro C, et al. Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb Haemost 2005; 93:1147–1152. [DOI] [PubMed] [Google Scholar]
- 14.Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. JTH 2010; 8:237–242. [DOI] [PubMed] [Google Scholar]
- 15.Elefsiniotis IS, Diamantis ID, Dourakis SP, et al. Anticardiolipin antibodies in chronic hepatitis B and chronic hepatitis D infection, and hepatitis B-related hepatocellular carcinoma. Relationship with portal vein thrombosis. Eur J Gastroenterol Hepatol 2003; 15:721–726. [DOI] [PubMed] [Google Scholar]
- 16.Gervais A, Czernichow B, Grunebaum L, et al. Serum cardiolipin antibodies in patients with alcoholic cirrhosis. Gastroenterol Clin Biol 1996; 20:736–742. [PubMed] [Google Scholar]
- 17.Egesel T, Buyukasik Y, Dundar SV, et al. The role of natural anticoagulant deficiencies and factor V Leiden in the development of idiopathic portal vein thrombosis. J Clin Gastroenterol 2000; 30:66–71. [DOI] [PubMed] [Google Scholar]
- 18.Rebours V, Boudaoud L, Vullierme MP, et al. Extrahepatic portal venous system thrombosis in recurrent acute and chronic alcoholic pancreatitis is caused by local inflammation and not thrombophilia. Am J Gastroenterol 2012; 107:1579–1585. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal R, Ravishankar B, Misra R, et al. Significance of elevated IgG anticardiolipin antibody levels in patients with Budd–Chiari syndrome. Am J Gastroenterol 1998; 93:954–957. [DOI] [PubMed] [Google Scholar]
- 20.Amitrano L, Ames PR, Guardascione MA, et al. Antiphospholipid antibodies and antiphospholipid syndrome: role in portal vein thrombosis in patients with and without liver cirrhosis. Clin Appl Thromb Hemost 2011; 17:367–370. [DOI] [PubMed] [Google Scholar]
- 21.Amitrano L, Anna Guardascione M, Brancaccio V, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004; 40:736–741. [DOI] [PubMed] [Google Scholar]
- 22.Amitrano L, Brancaccio V, Guardascione MA, et al. High prevalence of thrombophilic genotypes in patients with acute mesenteric vein thrombosis. Am J Gastroenterol 2001; 96:146–149. [DOI] [PubMed] [Google Scholar]
- 23.Amitrano L, Brancaccio V, Guardascione MA, et al. Portal vein thrombosis after variceal endoscopic sclerotherapy in cirrhotic patients: role of genetic thrombophilia. Endoscopy 2002; 34:535–538. [DOI] [PubMed] [Google Scholar]
- 24.Amitrano L, Guardascione MA, Brancaccio V, et al. Inherited trombophilic disorders in patients with portal vein thrombosis. J Hepatol 2000; 32 suppl 2:133. [DOI] [PubMed] [Google Scholar]
- 25.Egesel T, Unsal I, Bayraktar Y. Antiphospholipid antibodies and lipoprotein (a) as etiologic or contributory factors in patients with idiopathic cavernous transformation of portal vein. Turk J Gastroenterol 2002; 13:89–93. [PubMed] [Google Scholar]
- 26.Gulcan EM, Kutlu T, Erkan T, et al. Anticardiolipin antibodies in children with portal vein thrombosis. Turk Pediatri Arsivi 2009; 44:124–126. [Google Scholar]
- 27.Oksuzoglu G, Bayraktar Y, Arslan S, et al. Portal vein thrombosis in cirrhotics: related with anticardiolipin antibodies? Hepatogastroenterology 2003; 50:1527–1530. [PubMed] [Google Scholar]
- 28.Romero Gomez M, Suarez Garcia E, Lopez Lacomba D, et al. Antiphospholipid antibodies are related to portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol 2000; 31:237–240. [DOI] [PubMed] [Google Scholar]
- 29.Violi F, Ferro D, Basili S, et al. Increased rate of thrombin generation in hepatitis C virus cirrhotic patients. Relationship to venous thrombosis. J Investig Med 1995; 43:550–554. [PubMed] [Google Scholar]
- 30.Violi F, Ferro D, Basili S, et al. Relation between lupus anticoagulant and splanchnic venous thrombosis in cirrhosis of the liver. BMJ 1994; 309:239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yachha SK, Aggarwal R, Sharma BC, et al. Functional protein C and anti-cardiolipin antibody in children with portal vein thrombosis. Indian J Gastroenterol 2001; 20:47–49. [PubMed] [Google Scholar]
- 32.Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 2009; 51:682–689. [DOI] [PubMed] [Google Scholar]
- 33.Qi X, Ren W, De Stefano V, et al. Associations of coagulation factor V Leiden and prothrombin G20210A mutations with Budd–Chiari syndrome and portal vein thrombosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014; 12:1801–1812. [DOI] [PubMed] [Google Scholar]
- 34.Qi X, Yang Z, De Stefano V, et al. Methylenetetrahydrofolate reductase C677T gene mutation and hyperhomocysteinemia in Budd–Chiari syndrome and portal vein thrombosis: a systematic review and meta-analysis of observational studies. Hepatol Res 2014; 44:E480–E498. [DOI] [PubMed] [Google Scholar]
- 35.Qi X, De Stefano V, Wang J, et al. Prevalence of inherited antithrombin, protein C, and protein S deficiencies in portal vein system thrombosis and Budd–Chiari syndrome: a systematic review and meta-analysis of observational studies. J Gastroenterol Hepatol 2013; 28:432–442. [DOI] [PubMed] [Google Scholar]
- 36.Qi X, Yang Z, Bai M, et al. Meta-analysis: the significance of screening for JAK2V617F mutation in Budd–Chiari syndrome and portal venous system thrombosis. Aliment Pharmacol Ther 2011; 33:1087–1103. [DOI] [PubMed] [Google Scholar]
- 37.Rolla R, Vay D, Mottaran E, et al. Antiphospholipid antibodies associated with alcoholic liver disease specifically recognise oxidised phospholipids. Gut 2001; 49:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biron C, Andreani H, Blanc P, et al. Prevalence of antiphospholipid antibodies in patients with chronic liver disease related to alcohol or hepatitis C virus: correlation with liver injury. J Lab Clin Med 1998; 131:243–250. [DOI] [PubMed] [Google Scholar]
- 39.Yuste JR, Prieto J. Anticardiolipin antibodies in chronic viral hepatitis. Do they have clinical consequences? Eur J Gastroenterol Hepatol 2003; 15:717–719. [DOI] [PubMed] [Google Scholar]
- 40.Prieto J, Yuste JR, Beloqui O, et al. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology 1996; 23:199–204. [DOI] [PubMed] [Google Scholar]
- 41.Ordi-Ros J, Villarreal J, Monegal F, et al. Anticardiolipin antibodies in patients with chronic hepatitis C virus infection: characterization in relation to antiphospholipid syndrome. Clin Diagn Lab Immunol 2000; 7:241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangia A, Margaglione M, Cascavilla I, et al. Anticardiolipin antibodies in patients with liver disease. Am J Gastroenterol 1999; 94:2983–2987. [DOI] [PubMed] [Google Scholar]
- 43.Harada M, Fujisawa Y, Sakisaka S, et al. High prevalence of anticardiolipin antibodies in hepatitis C virus infection: lack of effects on thrombocytopenia and thrombotic complications. J Gastroenterol 2000; 35:272–277. [DOI] [PubMed] [Google Scholar]


