Supplemental Digital Content is available in the text
Abstract
The objective of this work is to demonstrate the potential time and labor savings that may result from increased use of combination vaccinations.
The study (GSK study identifier: HO-12-4735) was a model developed to evaluate the efficiency of the pediatric vaccine schedule, using time and motion studies. The model considered vaccination time and the associated labor costs, but vaccination acquisition costs were not considered. We also did not consider any efficacy or safety differences between formulations. The model inputs were supported by a targeted literature review. The reference year for the model was 2012.
The most efficient vaccination program using currently available vaccines was predicted to reduce costs through a combination of fewer injections (62%) and less time per vaccination (38%). The most versus the least efficient vaccine program was predicted to result in a 47% reduction in vaccination time and a 42% reduction in labor and supply costs. The estimated administration cost saving with the most versus the least efficient program was estimated to be nearly US $45 million. If hypothetical 6- or 7-valent vaccines are developed using the already most efficient schedule by adding additional antigens (pneumococcal conjugate vaccine and Haemophilus influenzae type b) to the most efficient 5-valent vaccine, the savings are predicted to be even greater.
Combination vaccinations reduce the time burden of the childhood immunization schedule and could create the potential to improve vaccination uptake and compliance as a result of fewer required injections.
INTRODUCTION
The number of recommended childhood vaccines has increased greatly over time. Childhood immunization based on the 2012 US-recommended schedule involved vaccinations against 11 diseases, with as many as 27 vaccinations by age 2 (Supplementary Figure 1, http://links.lww.com/MD/A127).1 The prospect of up to 7 vaccines at each visit, if combination vaccines are not used for infants, can be stressful for infants, parents, and providers.2–4
Combination vaccines combine several antigens against different diseases into a single injection or can incorporate several strains of agents causing one disease. Therefore, combination vaccines can reduce the number of injections. Combination vaccines have been in use for many years in the United States. There is a recognized unmet need for more combination vaccines to help alleviate the practical constraints of multiple injections, particularly for children who are behind schedule, and overcome the limited space in the office fridge of a physician. In a joint policy statement issued in 1999, the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP) stated a preference for the use of combination vaccines to reduce the number of injections at a single outpatient visit.5
This study aimed to demonstrate the potential time and labor savings that may result from the increased use of combination vaccinations and formulations that require less preparation. Specifically, this study quantified the impact of moving from the least to the most efficient vaccination program, as defined by the time and labor costs associated with the number of vaccine injections. The study also examined the potential of hypothetical multivalent combinations. The study does not review the difference in acquisition prices between different presentations of vaccines or combinations to avoid obscurities of analysis.
MATERIALS AND METHODS
Time Required per Vaccination
A targeted review of time–motion studies published between January 2002 and June 2012 was conducted. We reviewed MEDLINE-indexed publications and a supplementary search of the ‘gray’ literature (Supplementary Data, http://links.lww.com/MD/A127, Supplementary Table 1, http://links.lww.com/MD/A127 and Supplementary Figure 2, http://links.lww.com/MD/A127).6–16
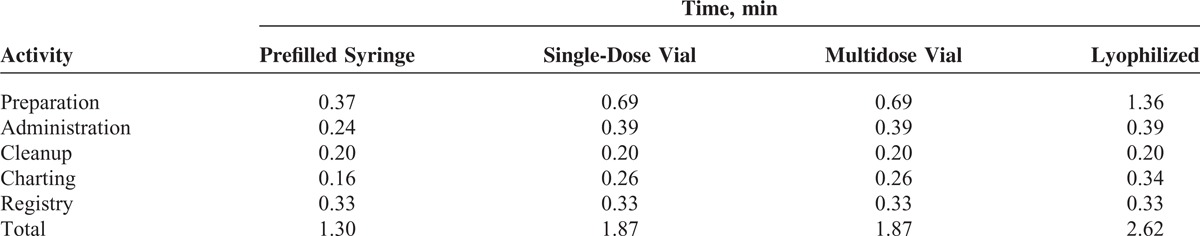
The details of the estimates and assumptions used in the model can be referred to in the Supplementary Data, http://links.lww.com/MD/A127. All of the estimates used in the model for vaccination-related activities are shown in Table 1.
TABLE 1.
Times Used for Vaccine-Related Activities
Model Overview
The model was developed to evaluate the efficiency of physician practices in their vaccination programs for children aged 0 to 6 years (GSK study identifier: HO-12-4735). Efficiency was measured in terms of time devoted to vaccinations and the associated labor costs. Activity-based costing was selected as the modeling technique because it captures the impact of dose form (eg, lyophilized, single vial, etc) and monovalent versus multivalent vaccinations on each aspect of the program. The efficacy of monovalent and multivalent vaccinations were assumed the same as no clinical information was considered; therefore, efficacy and safety cannot be derived regarding any product or presentation on the basis of this model. However, the impact of single antigen versus combination vaccination immunogenicity on cost-effectiveness (which was beyond the scope of this analysis) is an area that warrants further research.
The model computed the following measures of efficiency: total number of vaccinations, total time spent on vaccinations, total labor and supply costs (only ancillary items, refer to Supplementary Data, http://links.lww.com/MD/A127) for vaccinations, average time per vaccination, and average labor and supply cost per vaccination. Vaccine acquisition costs and vaccination administration fees (physician reimbursement) were not included, as the authors sought to investigate the impact of combination use and vaccine presentation type free of these 2 elements.
The model calculated vaccine utilization for 2012 and required 3 data inputs: population numbers and age distribution, vaccination schedule, and time per vaccine by dose form.
For physician practices and the patient population information, refer to the Supplementary Data, http://links.lww.com/MD/A127, Supplementary Tables 2 and 3, http://links.lww.com/MD/A127.
Schedule of Vaccines Used by Providers
The model assumed that the vaccination schedule conforms to the recommended immunization schedule for persons aged 0 to 6 years, approved by the ACIP, AAP, and AAFP.1
Costs
The literature was reviewed in order to derive cost data on consumables used in vaccine administration. Three publications in Supplementary Table 4, http://links.lww.com/MD/A127 included consumable cost information6,9,10 (Supplementary Table 5, http://links.lww.com/MD/A127), and these provided similar values. The study by Glazner et al10 was used, as it provided the most recent information, and it was a US-based study. Disposable cost estimates were inflated to 2012 international dollars using the consumer price index.17
Hourly labor costs were taken to be $81.08 for pediatricians and $33.23 for nurses.18
Scenario Analysis Overview
The activity-based costing model was used to examine vaccination program efficiency with currently available products and the potential impact of hypothetical higher combination multivalent vaccinations in future. The efficiency of current vaccination programs was examined by determining the impact of moving from the least to the most efficient vaccination program. When calculating efficiency, the following limitations apply: no assumptions on vaccination efficacy or safety were made and vaccination catch-up requirements were not considered; vaccination programs are limited to children aged 0 to 6 years; analysis assumes that physicians adhere to vaccination recommendations and that there is sufficient vaccine supply; reimbursement implications of combination vaccinations are not included.
Least Efficient Vaccination Program
The following principles were used to define the least efficient vaccination program (the greatest total time spent on vaccination-related activities): maximum total number of vaccinations (distinct injections/applications); monovalent vaccinations were selected over multivalent vaccinations, and maximum time per vaccination (preference by form using the following order: lyophilized, multivial, single vial, prefilled syringe). The schedule for the least efficient vaccination program is provided in Supplementary Figure 3, http://links.lww.com/MD/A127.
Most Efficient Vaccination Program
The following guiding principles were used to define the most efficient program (least total time spent on vaccination-related activities): minimum total number of vaccinations (multivalent vaccinations were selected over monovalent vaccines; preference was given for higher versus lower valent combination vaccinations) and minimum time per vaccination [preference by form using the following order: prefilled syringe, single vial, multivial, and lyophilized].
Using currently available vaccines, the aforementioned criteria yielded 3 possible candidates for the most efficient program: Pediarix™ (GlaxoSmithKline Biologicals, Rixensart, Belgium), a pentavalent conjugate vaccine containing diphtheria, tetanus, and acellular pertussis (DTaP)-Hepatitis B-inactivated poliovirus vaccine (IPV); Pentacel™ (Sanofi Pasteur Inc, Swiftwater, PA), a conjugate vaccine for DTaP-IPV-Haemophilus influenzae type b (Hib) or a combination of Infanrix™ (GlaxoSmithKline Biologicals) a DTaP conjugate vaccine; and Comvax™ (Merck and Co, Inc, Whitehouse Station, NJ) indicated for immunization against Hib and Hepatitis B.
Iterative testing using the activities-based costing model was performed to determine the most efficient program, which is provided in Supplementary Figure 4, http://links.lww.com/MD/A127.
Pediarix™ was found to be the most cost-effective as the number of diseases prevented with a single injection were greater. Pediarix™ did need to be reconstituted unlike Pentacel™.
An Infanrix™/Comvax™ schedule resulted in 4 additional injections over the Pediarix™- and Pentacel™-based schedules. When comparing the Pediarix™- and Pentacel™-based schedules, although the number of injections/applications are the same, the difference in formulation (prefilled syringes versus lyophilized) resulted in additional efficiencies for a Pediarix™-related program due to timesaving (5.8 min/child; ∼183,000 hours overall) and reduced labor costs ($3.22 per child; US $6.1 million overall). Therefore, Pediarix™ was used in the most efficient schedule calculations and as the basis for the hypothetical 6- and 7-valent analyses.
Additional Analyses
A sensitivity analysis was performed in which preparation time was made equivalent at 0.70 minutes for prefilled syringe, single dose vial, and multidose vial, whereas lyophilized remain unchanged; administration and charting times were made equivalent at 0.39 and 0.34 minutes respectively for all formulations. In addition, the impact of a hypothetical increase in the number of diseases is covered by a single combination vaccine, using the most efficient vaccination program utilizing the most efficient 5-valent vaccine as the core and adding additional antigens to this 5-valent vaccine, thereby, reducing the number of injections administered. In the first scenario, a hypothetical 6-valent vaccination was selected, by adding pneumococcal conjugate vaccine (PCV) to DTaP-Hepatitis B-IPV (Pediarix™). PCV was selected for inclusion in the 6-valent scenario because it resulted in fewer total vaccinations (individual discrete injections) than other vaccination types. A 6-valent vaccine with PCV could potentially yield greater timesavings than one with Hib (10% versus 7% timesavings); therefore, it was selected as the sixth antigen. The second scenario created a 7-valent vaccination that added PCV and Hib to DTaP-Hepatitis B-IPV (Pediarix™). These vaccination programs can be found in Supplementary Figures 5 and 6, http://links.lww.com/MD/A127.
RESULTS
Currently Available Formulations
For children aged 0 to 6 years, the most efficient vaccine program was predicted to reduce costs through a combination of fewer vaccinations (individual discrete injections/applications), less time per vaccination, and lower labor and supply costs. Most of the timesaving was attributed to fewer injections (62%), which was attributed to the use of combination vaccines, with the other 38% attributed to less time spent per vaccination visit was interrelated to the use of prefilled formulations. Annually, this is estimated to result in a 29% reduction in the number of individual vaccinations required (potential savings = 18,291,654); a 47% reduction in vaccination time (>1 million hours of pediatrician/nurse time saved); and a 42% reduction in labor and supply costs (totaling nearly US $45 million saved) (Table 2).
TABLE 2.
Annual Comparison of the Most and Least Efficient Currently Available Vaccination Programs
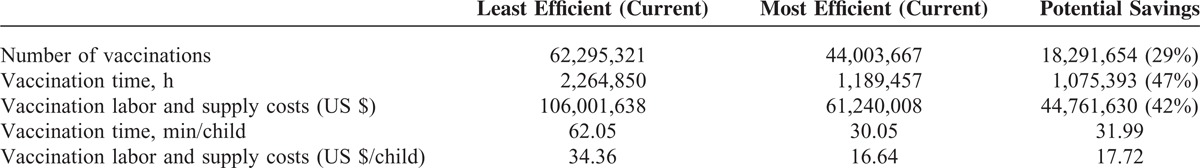
Figure 1 shows a per-vaccination activity-based view of the 2 programs. The majority of the time saved per vaccination was the result of less time spent in preparation activities; for example, a prefilled syringe requires less preparation time than a lyophilized or multivial vaccination. Total time saved per vaccination was 0.56 minutes. This results in per-child savings of 16.5 minutes and $11.35, respectively over the duration of the first 6 years of the pediatric vaccination schedule.
FIGURE 1.
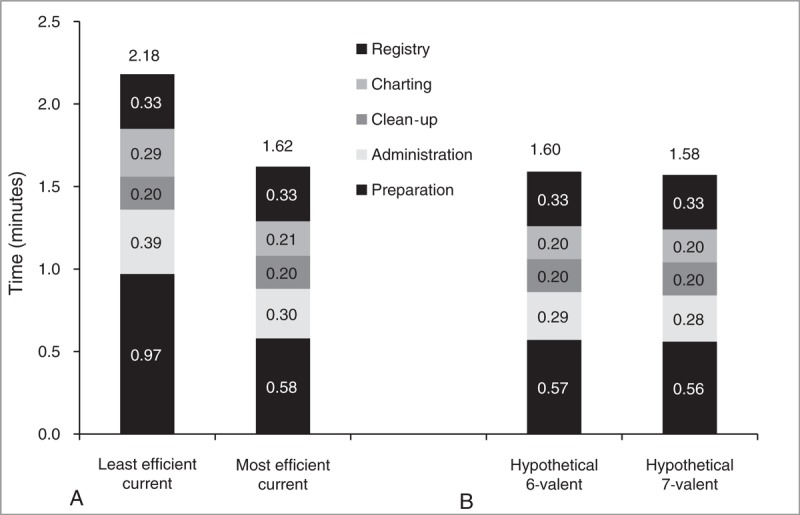
Per vaccination activity-based view of the vaccination programs.
Efficiency gains were reduced in the sensitivity analysis [the same preparation time for prefilled syringe, single dose, and multidose vial; the same charting and administration times across all formulations] with a 35% reduction in vaccination time (818,000 hours of pediatrician/nurse-time saved); and a 34% reduction in labor and supply costs (totaling nearly US $36 million saved).
Hypothetical Formulations
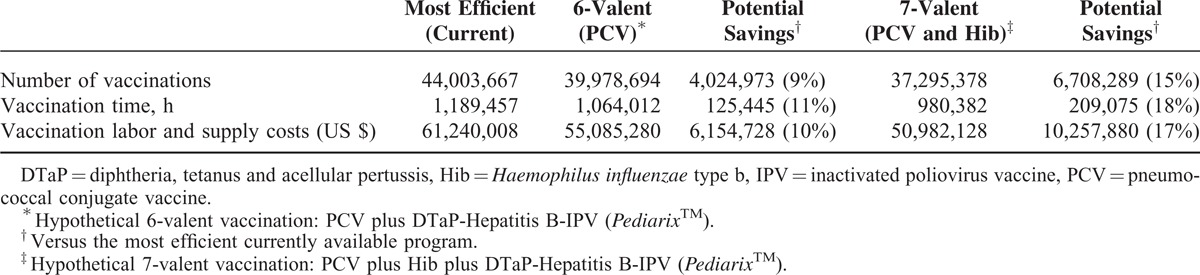
Table 3 shows potential additional annual timesavings of 125,445 hours (11%) with a hypothetical 6-valent and 209,075 hours (18%) with a hypothetical 7-valent vaccination, compared with the most efficient current schedule. Using 6- or 7-valent vaccines is predicted to result in annual labor and supply cost savings of US $6 million (10%) or US $10 million (17%), respectively. Figure 1 shows a per-vaccination activity-based view of the 2 hypothetical vaccination programs. An alternative 6-valent formulation was also considered, using Hib instead of PCV, but this was less favorable, resulting in an additional 41,815 hours and US $1,389,512 over the hypothetical 6-valent PCV containing formulation.
TABLE 3.
Annual Cost Comparison of the Current Most Efficient Schedule and Hypothetical 6-Valent and 7-Valent Vaccination Programs
DISCUSSION
This model, even though limited by data constraints, predicted that using the most time-efficient vaccinations currently available could reduce the number of injections by 29%, decrease the time spent on vaccinations by 47%, and save nearly US $45 million in labor and supply costs, compared with the least efficient option. The main reason for these savings was due to maximizing the use of a maximum number of combination vaccines. These could include Pediarix™, Pentacel™, or ProQuad™ (Merck and Co, Inc). Furthermore, the use of PedvaxHIB™ (Hib; Merck and Co, Inc) rather than ActHIB™ (Hib; Sanofi Pasteur Inc) and Rotarix™ (rotavirus; GlaxoSmithKline Biologicals) rather than RotaTeq™ (rotavirus; Merck and Co, Inc) saved injections/oral applications as only 2, rather than 3, doses are required in the initial series.
Pediarix™ and Pentacel™ are 5-valent vaccines. Pediarix™ is provided as a prefilled syringe, whereas Pentacel™ is a lyophilized formulation. The inclusion of either of these 5-valent vaccines leads to the fewest number of vaccine applications and requires the same number of vaccination injections. However, further timesaving can be realized by including vaccinations that are quicker and easier to administer, such as prefilled syringes. It is for this reason that the time and labor costs saved, when the program utilized Pediarix™ rather than Pentacel™, amounted to between $4.3 and $6.1 million. Therefore, a vaccination program that includes Pediarix™ is the most efficient schedule.
Further analysis demonstrated the potential for additional timesavings by the use of higher combination multivalent vaccinations. Specifically, combinations of Pediarix™ with PCV and/or Hib, both of which require frequent injections, offer the potential for additional timesavings.
In addition to the labor and cost savings, combination vaccines have many potential benefits, which in some way together with their reduced impact on parents and children might assist in justifying that their acquisition costs are generally higher than the sum of acquisition cost of single presentation. Fewer injections are obviously more beneficial, reducing discomfort and anxiety.2,4,19 Not surprisingly, combination vaccines are generally preferred by parents, as fewer injections are required.4,20–24 They can also result in fewer physician visits, saving time and money.3,4 Combination vaccines can also improve the efficiency of physician practices by reducing the need to stock and administer separate vaccines, the number of visits required, health care provider workload, and simplifying of record keeping.2,3,25 Furthermore, the use of combination vaccines should improve vaccine uptake, compliance, and timeliness.2,25–27 This has the potential to reduce the incidence of vaccine-preventable disease, thus, saving morbidity, mortality, and treatment costs. Despite concerns that the immune system may be overloaded with combination vaccines, this appears not to be the case.28 Similarly, concerns about more serious adverse events resulting from the use of combination vaccines appear to be overestimated.2
However, there are various barriers to the use of combination vaccines. Firstly, they tend to be more expensive than the sum of their constituent parts.27 Secondly, as health care providers receive administration fees per injection given, the use of combination vaccines can potentially reduce their income.2,25,27,29 However, the time saved in giving vaccinations can usefully be used for other activities. Thirdly, using combination vaccines can result in additional doses being given.25 For example, if Pediarix™ is given at 2, 4, and 6 months, it results in an additional hepatitis B dose at 4 months. However, this has been found not to be detrimental,30 although data are limited, and approved by the Centers for Disease Control and Prevention.1 Lastly, whereas combination vaccines have been suggested to reduce the need to stock separate vaccines,3,25 if they do not completely replace single vaccines, this would result in more products having to be stocked.19
As with any study, this current analysis has various limitations. The focus of this study was on the efficiency of a provider's practice at administering vaccinations, so other factors that may impact the actual administration were not considered, for example, the efficacy and safety of the vaccinations, and for simplification purposes, these were assumed to be the same for single antigen vaccines and combination vaccines. The impact of single antigen versus combination vaccination on cost-effectiveness (which was beyond the scope of this analysis) is also an area that warrants further research. Also, estimates used were limited to current literature, which might not accurately reflect practice due to electronic medical records and barcodes, or costs of biohazard waste removal. The reimbursement implications of combination vaccinations are not included in the model; nor did it consider the differences in vaccination location, that is, vaccination clinic versus physician's office. Moreover, the costs considered were limited to the administration costs only and did not include the costs of the different vaccines, so this study does not assess the impact on overall health care costs.
In conclusion, combination vaccinations can reduce the time burden of the childhood immunization schedule and create the potential to improve vaccination uptake and compliance as a result of fewer required injections for children. For physician practices, combination vaccines can improve office efficiency. These results highlight an opportunity for physicians to improve their practice efficiency related to vaccination through the incorporation of combination vaccinations and dosage forms that require fewer administration steps.
Acknowledgments
We would like to thank Karen Sandman for her assistance with literature review and method selection. We also thank Dr Jenny Lloyd (freelance medical writer on behalf of GSK), Preethi Govindarajan (publication writer, GSK) who provided medical writing and editorial assistance, Heather Santiago (publication manager, GSK), Jérémie Dedessus le Moutier and Grégory Leroux (publication manager, Business & Decision Life Sciences on behalf of GSK Vaccines) for editorial assistance and article coordination.
Trademark Statements
Pediarix™, Rotarix™, and Infanrix™ are trademarks of the GSK group of companies.
Pentacel™ and ActHIB™ are trademarks of Sanofi Pasteur Inc.
ProQuad™, PedvaxHIB™, RotaTeq™, and Comvax™ are trademarks of Merck and Co, Inc.
Footnotes
Abbreviations: DTaP = diphtheria, tetanus, and acellular pertussis, Hib = Haemophilus influenzae type b, IPV = inactivated poliovirus vaccine, PCV = pneumococcal conjugate vaccine.
All authors participated in the design, or implementation, or analysis, and interpretation of the study and the development of this article. All authors had full access to the data and gave final approval before submission. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. GM wrote the first draft of the article. Jenny Lloyd (freelance medical writer on behalf of GSK) received payment for medical writing services at the direction of the authors to develop the drafts.
GlaxoSmithKline Biologicals SA funded this study/research and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took the responsibility of all costs associated with the development and publication of this article.
MC, SEB, JG, and BH declare that their former institution, Evidera (formerly United BioSource Corporation), received funding from the GSK group of companies to complete part of the work disclosed in this article (model development, statistical analysis, and scientific input). GM and BH are employed by the GSK group of companies and have restricted shares in the GSK group of companies. All authors declare no other conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0–18 years—United States, 2012. Morb Mortal Wkly Rep 2012; 61:1–4. [PubMed] [Google Scholar]
- 2.Goldfarb NI, Patel NM, Clarke JL. Improving quality by encouraging providers to use pediatric combination vaccines. Manag Care 2005; 14 (6 suppl):3–12. [PubMed] [Google Scholar]
- 3.Koslap-Petraco MB, Parsons T. Communicating the benefits of combination vaccines to parents and health care providers. J Pediatr Health Care 2003; 17:53–57. [DOI] [PubMed] [Google Scholar]
- 4.Dodd D. Benefits of combination vaccines: effective vaccination on a simplified schedule. Am J Manag Care 2003; 9 (1 suppl):S6–S12. [PubMed] [Google Scholar]
- 5.Centres for Disease Control, Prevention (CDC). Recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). MMWR Recomm Rep 1999; 48:1–14. [Google Scholar]
- 6.Turner N, Rouse P, Airey S, et al. The cost of immunising at the general practice level. J Prim Health Care 2009; 1:286–296. [PubMed] [Google Scholar]
- 7.Szilagyi PG, Iwane MK, Humiston SE, et al. Time spent by primary care practices on pediatric influenza vaccination visits: implications for universal influenza vaccination. Arch Pediatr Adolesc Med 2003; 157:191–195. [DOI] [PubMed] [Google Scholar]
- 8.Wiedenmayer KA, Weiss S, Chattopadhyay C, et al. Simplifying paediatric immunization with a fully liquid DTP-HepB-Hib combination vaccine: evidence from a comparative time–motion study in India. Vaccine 2009; 27:655–659. [DOI] [PubMed] [Google Scholar]
- 9.Glazner JE, Beaty BL, Pearson KA, et al. The cost of giving childhood vaccinations: differences among provider types. Pediatrics 2004; 113:1582–1587. [DOI] [PubMed] [Google Scholar]
- 10.Glazner JE, Beaty B, Berman S. Cost of vaccine administration among pediatric practices. Pediatrics 2009; 124 suppl 5:S492–S498. [DOI] [PubMed] [Google Scholar]
- 11.Glazner JE, Beaty BL, Pearson KA, et al. Using an immunization registry: effect on practice costs and time. Ambul Pediatr 2004; 4:34–40. [DOI] [PubMed] [Google Scholar]
- 12.Yoo BK, Szilagyi PG, Schaffer SJ, et al. Cost of universal influenza vaccination of children in pediatric practices. Pediatrics 2009; 124 suppl 5:S499–S506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyay A, Ghosh R, Maji S, et al. A time motion study in the immunization clinic of a tertiary care hospital of Kolkata, West Bengal. Indian J Community Med 2012; 37:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-lela OQ, Bahari MB, Al-abbassi MG, et al. Estimation of immunization providers’ activities cost, medication cost, and immunization dose errors cost in Iraq. Vaccine 2012; 30:3862–3866. [DOI] [PubMed] [Google Scholar]
- 15.Katz SL, Klien JO, et al. Increasing influenza immunization rates in infants and children: putting recommendations into practice. NFID. 2003. p. 6–7 [Google Scholar]
- 16.Manzi F, Schellenberg J, Hamis Y, et al. Intermittent preventive treatment for malaria and anaemia control in Tanzanian infants; the development and implementation of a public health strategy. Trans R Soc Trop Med Hyg 2009; 103:79–86. [DOI] [PubMed] [Google Scholar]
- 17.Consumer price index for medical care services. Bureau of Labor Statistics, 2012. http://www.bls.gov/cpi/cpid1212.pdf Accessed June 3, 2014. [Google Scholar]
- 18.Occupational Employment Statistics. Occupation Profiles. Bureau of Labor Statistics, 2011. http://www.bls.gov/oes/current/oes_stru.htm Accessed June 3, 2014. [Google Scholar]
- 19.Le CT. Combination vaccines: choices or chaos? A practitioner's perspective. Clin Infect Dis 2001; 33 suppl 4:S367–S371. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhoff AS, Weniger BG, Jacobs RJ. Economic value to parents of reducing the pain and emotional distress of childhood vaccine injections. Pediatr Infect Dis J 2001; 20 suppl 11:S57–S62. [DOI] [PubMed] [Google Scholar]
- 21.Freed GL, Cowan AE, Clark SJ, et al. Use of a new combined vaccine in pediatric practices. Pediatrics 2006; 118:e251–e257. [DOI] [PubMed] [Google Scholar]
- 22.Gidengil C, Lieu TA, Payne K, et al. Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 2012; 30:3445–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theeten H, Hens N, Aerts M, et al. Common attitudes about concomitant vaccine injections for infants and adolescents in Flanders, Belgium. Vaccine 2009; 27:1964–1969. [DOI] [PubMed] [Google Scholar]
- 24.Theeten H, Hens N, Aerts M, et al. Caregivers’ willingness to pay to reduce the number of vaccine injections in infants. Pediatr Infect Dis J 2009; 28:61–63. [DOI] [PubMed] [Google Scholar]
- 25.Koslap-Petraco MB, Judelsohn RG. Societal impact of combination vaccines: experiences of physicians, nurses, and parents. J Pediatr Health Care 2008; 22:300–309. [DOI] [PubMed] [Google Scholar]
- 26.Kalies H, Grote V, Verstraeten T, et al. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 2006; 25:507–512. [DOI] [PubMed] [Google Scholar]
- 27.Gidengil CA, Rusinak D, Allred NJ, et al. Financial barriers to implementing combination vaccines: perspectives from pediatricians and policy makers. Clin Pediatr 2009; 48:539–547. [DOI] [PubMed] [Google Scholar]
- 28.Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant's immune system? Pediatrics 2002; 109:124–129. [DOI] [PubMed] [Google Scholar]
- 29.Shen AK, Sobczyk E, Simonsen L, et al. Financial impact to providers using pediatric combination vaccines. Pediatrics 2011; 128:1087–1093. [DOI] [PubMed] [Google Scholar]
- 30.Pichichero ME, Blatter MM, Reisinger KS, et al. Impact of a birth dose of hepatitis B vaccine on the reactogenicity and immunogenicity of diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b combination vaccination. Pediatr Infect Dis J 2002; 21:854–859. [DOI] [PubMed] [Google Scholar]


