FIGURE 1.
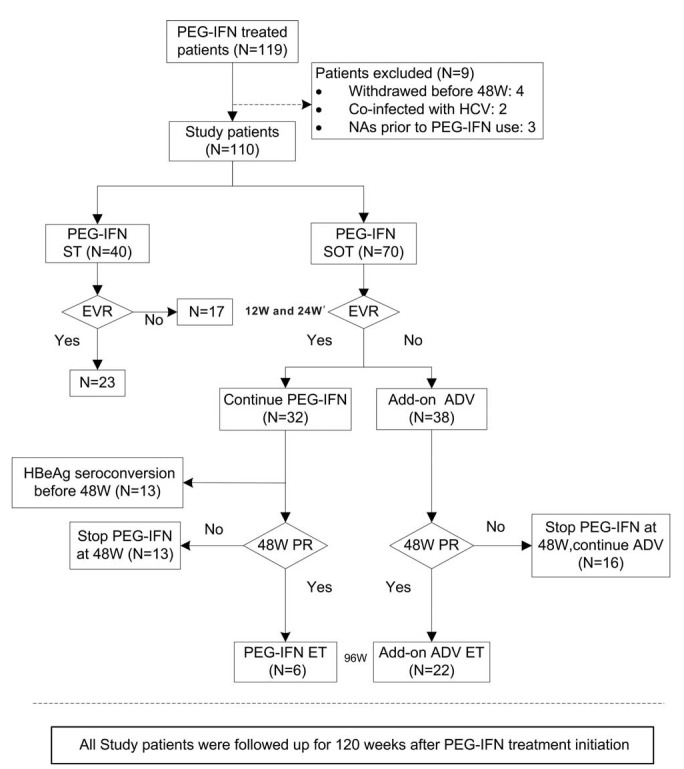
Flow chart of the study population and design. EVR, including 12-week EVR (12W EVR) and 24-week EVR (24W EVR) in our study at two time points. 12W EVR was defined as at least a 2 log10 reduction from baseline in HBV DNA copies/mL at week 12. Twenty-four-week EVR was defined as at least a 1 log10 reduction from baseline in HBsAg IU/mL or HBsAg < 1500 IU/mL at week 24. The 48W PR (48-week partial response) was defined as 1.0 ≤ HBeAg ≤ 10.0 S/CO or HBeAg >10.0 S/CO but HBsAg <1000 IU/mL at week 48. ADV = adefovir, ET = extended treatment, EVR = early virological response, HBeAg = hepatitis B virus e antigen, HCV = hepatitis C virus, NAs = nucleot(s)ide analogues, PEG-IFN SOT = pegylated interferon-α-2a stepwise optimization treatment, PEG-IFN ST = pegylated interferon-α-2a standard therapy, PR = partial response.
