Fig. 2.
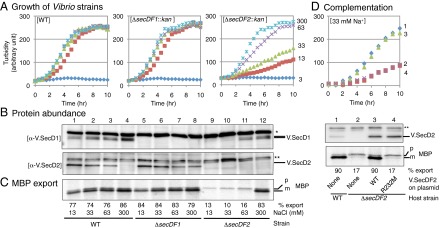
Physiological roles of V.SecDF1 and V.SecDF2 in V. alginolyticus. (A) V.SecDF2 is important for growth under Na+-limited conditions. WT and the indicated deletion mutants of V. alginolyticus were grown at 30 °C in M63 medium supplemented with a total of 300 mM of NaCl and KCl, in which Na+ concentrations were 3 (blue), 13 (brown), 33 (pale green), 63 (purple), and 300 (pale blue) mM. Cell densities were monitored by turbidity measurements. (B) Cellular abundance of V.SecD2 correlates inversely with Na+ concentrations and the presence of V.SecDF1. V. alginlyticus strains indicated at the bottom of C were grown at 30 °C until a mid log phase in the media supplemented with the indicated concentrations of Na+. Total cellular proteins of equivalent amounts were separated by Laemmli SDS/PAGE, followed by immunodetection of V.SecD1 (Upper) and V.SecD2 (Lower). Asterisks show nonspecific background bands. (C) Severe protein export defects in the absence of V.SecDF2 and sufficient Na+ levels. The same Vibrio strains used in B were grown at 30 °C in M63 medium supplemented with the indicated concentrations of Na+ and pulse-labeled for 30 s. Extracts of equivalent radioactivities were used for immunoprecipitation of MBP. Upon electrophoresis, precursor (p) and mature (m) bands of MBP were quantified to give the efficiencies of processing (export) shown at the bottom. (D) Activities of V.SecDF2 to support growth and protein export. (Upper) Growth at 30 °C in M63 medium supplemented with 33 mM NaCl and 10 μM isopropyl β-D-1-thiogalactopyranoside (IPTG) was followed for the following V. alginolyticus strains: 1, WT (VIO5) carrying empty vector and 2–4, ΔsecDF2 carrying the vector (2), carrying the V.SecDF2-expressing plasmid (3), and carrying the V.SecDF2(R232M)-expressing plasmid (4). (Middle) Cellular abundance of V.SecD2 was examined by anti-V.SecD2 immunoblotting. Asterisks show nonspecific background signals. (Lower) Export of MBP was followed for the above strains by [35S]methionine pulse-labeling for 1 min at 30 °C. Processing efficiencies (percent) are shown at the bottom. See also Figs. S2 and S3.
