Fig. 7.
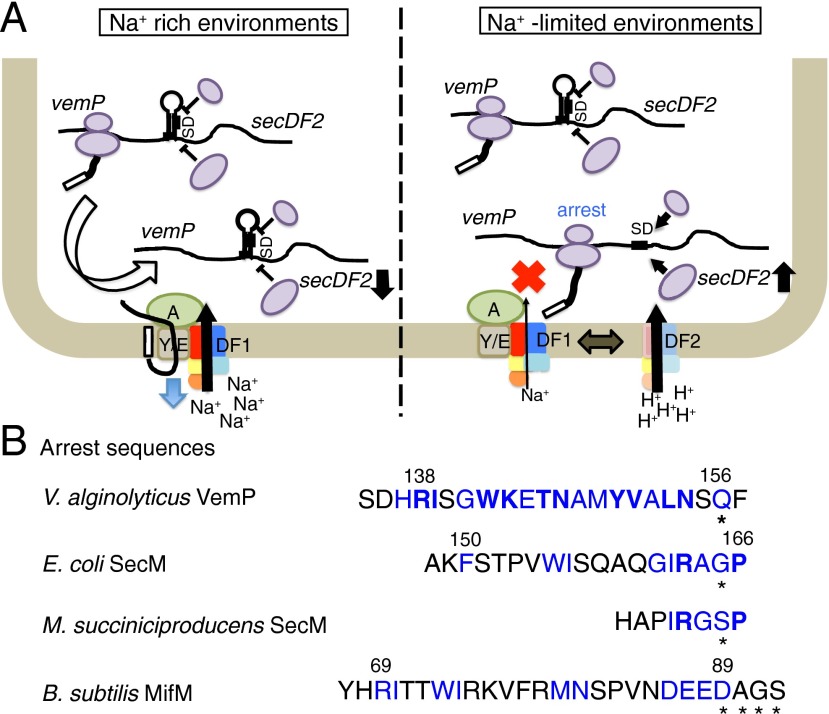
(A) Adaptation of Vibrio species to salinity changes through export-controlled translation arrest of VemP. (Left) Repression of V.SecDF2 synthesis in Na+-rich environments. Because the VemP export occurs efficiently by the V.SecDF1-containing Sec machinery capable of using the Na+-motive force, the VemP elongation arrest is transient. In this situation, the duration of the arrest, in which the Shine–Dalgarno sequence of V.SecD2 is available, is short, leading to low-level synthesis of V.SecD2. (Right) Up-regulation of V.SecDF2 synthesis in Na+-limited environments. In the absence of sufficient concentrations of Na+, activity of the V.SecDF1-containing Sec machinery will decline, leading to retarded export and prolonged elongation arrest of VemP. This in turn results in higher rates of initiation of secDF2VA translation. Newly synthesized V.SecDF2 molecules will substitute for V.SecDF1 such that the bacteria can now use the proton-motive force to effectively localize their secretory proteins. (B) Diversity in the intrinsic class of arrest sequences. Shown are the amino acid sequences that induce translation arrest without any cofactor, which have been elucidated for VemP, SecM (18, 52), and MifM (19). Residues whose identities contribute to the elongation arrest are highlighted in blue; particularly important residues are shown in boldface. Asterisks indicate the last residue of the arrested peptidyl-tRNA, which resides in the P-site of the ribosome. See also Fig. S6.