Significance
Dietary change among hominins is a critical aspect of human evolution. Here we use carbon isotope data from fossil teeth of hominins, monkeys, and other mammals from Ethiopia to document C4 food consumption by both hominins and the baboon, Theropithecus oswaldi, during the early Pliocene. The expansion of hominin diet and the appearance of the Theropithecus oswaldi lineage as early as 3.76 Ma mark a major ecological change within African primate communities. The ability to eat a range of C3 and C4 foods indicates that early Pliocene hominins were likely generalists who could thrive in different and perhaps varying environments.
Keywords: hominins, Woranso-Mille, Theropithecus, carbon isotopes, paleodiet
Abstract
The incorporation of C4 resources into hominin diet signifies increased dietary breadth within hominins and divergence from the dietary patterns of other great apes. Morphological evidence indicates that hominin diet became increasingly diverse by 4.2 million years ago but may not have included large proportions of C4 foods until 800 thousand years later, given the available isotopic evidence. Here we use carbon isotope data from early to mid Pliocene hominin and cercopithecid fossils from Woranso-Mille (central Afar, Ethiopia) to constrain the timing of this dietary change and its ecological context. We show that both hominins and some papionins expanded their diets to include C4 resources as early as 3.76 Ma. Among hominins, this dietary expansion postdates the major dentognathic morphological changes that distinguish Australopithecus from Ardipithecus, but it occurs amid a continuum of adaptations to diets of tougher, harder foods and to committed terrestrial bipedality. In contrast, carbon isotope data from cercopithecids indicate that C4-dominated diets of the earliest members of the Theropithecus oswaldi lineage preceded the dental specialization for grazing but occurred after they were fully terrestrial. The combined data indicate that the inclusion of C4 foods in hominin diet occurred as part of broader ecological changes in African primate communities.
The Pliocene is a critical time in human evolution when almost all early hominins became committed terrestrial bipeds and expanded their diets to include a wider range of resources than their ancestors. Recent stable isotope studies of Australopithecus afarensis teeth indicate that hominins had increased their dietary breadth to include significant amounts of C4 or crassulacean acid metabolism (CAM) resources by 3.4 Ma (1), which is in contrast to its putative ancestor, Australopithecus anamensis, whose diet was limited to predominantly C3 foods (2). The expansion in hominin diets to include significant amounts of C4 and CAM resources indicates a transition toward more open-country foods, because C3 plants include trees, shrubs, forbs, and cool-growing season grasses, whereas C4 plants are primarily warm-growing season grasses and sedges, and CAM plants include cacti and succulents (3). This dietary expansion may have made it easier for hominins to survive in a greater range of environments or in environments that were more variable. However, we do not know when hominins started to include large proportions of C4 resources in their diets, nor do we fully understand the ecological context of these changes (1, 4).
We use stable carbon isotope ratios of fossil hominin and cercopithecid teeth from Woranso-Mille in the western part of the central Afar Rift in Ethiopia (Fig. S1) to refine the timing of hominin dietary expansion to include C4 resources. We also report isotope data from other mammals and soil carbonates to evaluate the environmental context and the diagenetic integrity of the isotope data. We use stable carbon isotope ratios, or δ13C values, of fossil teeth to evaluate the dietary proportion of plants that use the C3 vs. C4 photosynthetic pathway, based on the premise that C3 and C4 plants have distinct carbon isotope signatures and that the δ13C value of tooth enamel reflects the carbon isotope composition of an animal’s diet (5). Fossil teeth from the Woranso-Mille paleontological study area are well-suited to fill the temporal gap in the isotopic record of hominin diet because they are part of a record of Pliocene mammalian fossils that spans 3.76–3.2 Ma (6–11). The hominin fossils from Woranso-Mille include those that are morphologically intermediate between Au. anamensis and Au. afarensis, some that are definitively Au. afarensis, and others that represent additional species (7–9, 12). The cercopithecids include multiple species of colobines and at least two papionins (10). Theropithecus oswaldi cf. darti is the most common cercopithecid in the assemblages (>90% of identifiable cercopithecid specimens; 40% of the total identifiable mammal specimens) (6, 10); it represents the oldest and most primitive representative of the long-lasting T. oswaldi lineage whose morphology became increasingly specialized for grazing throughout the Pliocene and into the Pleistocene (13). The carbon isotope data from cercopithecids sympatric to hominins at Woranso-Mille make it possible to evaluate the ecological context for dietary changes in hominins.
Fig. S1.
Map with locations of sites discussed in text and listed in Datasets S2 and S5. Woranso-Mille, Middle Awash (Aramis, Asa Issie, Asa Koma), Dikika, Gona (Segala Noumou), and Hadar and are in the Afar region in Ethiopia. Lomekwi, Allia Bay, and Kanapoi are in the Omo-Turkana region of Kenya. Imagery is from NASA's Earth Observatory from August 2004 (72).
Results
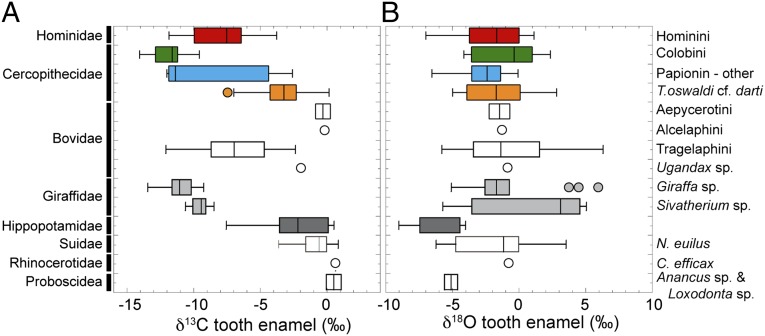
We report carbon and oxygen stable isotope data from 152 fossil teeth of hominins, cercopithecids, bovids, giraffids, hippopotamids, suids, rhinoceratids, and proboscidea from three time intervals at Woranso-Mille (3.76–3.57 Ma, 3.57–3.47 Ma, and 3.47–3.2 Ma; see Supporting Information for additional context). Among these teeth, δ13C values range from −14.0 to +1.1‰ and represent the full spectrum of δ13C values expected for tooth enamel from mammals with diets of exclusively C3 and exclusively C4 resources (Fig. 1 and Dataset S1). Presumed browsers such as giraffids and colobines yield δ13C values at the lower end of this range, whereas δ13C values of presumed grazers including alcelaphini, Loxodonta, Anancus, and Notochoerus euilus are at the high end of this range. These distributions suggest that the carbon isotope data from the fossil teeth at Woranso-Mille preserve dietary information and are not products of diagenesis. There are no significant differences in δ13C values among any of the mammalian taxa from the different time intervals sampled at Woranso-Mille (P > 0.05), such that we consider the fossil δ13C data from all of the time intervals together.
Fig. 1.
Box and whisker plots of δ13C (A) and δ18O (B) values for the fossil tooth enamel data from Woranso-Mille. Median values are marked by a vertical line within the box, the edges of the boxes represent quartile values, the horizontal lines indicate the range, and outliers are plotted as circles.
Hominins.
Sampling was limited to fragmentary hominin teeth whose genus and species cannot be determined with certainty; the sample may include intermediate forms of the Au. anamensis–Au. afarensis lineage, Au. afarensis, Australopithecus deyiremeda, and a yet unnamed species represented by the BRT-VP-2/73 foot (9, 12). The Woranso-Mille hominin teeth yield a mean δ13C of −7.9 ± 2.5‰ (1σ) and range from −11.8 to −3.8‰ (n = 16, median −7.5‰). The δ13C values of hominin teeth from Woranso-Mille are indistinguishable from that of non-Theropithecus papionins, Tragelaphus, sivatheres, and hippopatimds (P > 0.1), but distinct from the δ13C values of all other taxonomic groups sampled (P < 0.001). Notably, δ13C values of hominins at Woranso-Mille are significantly higher than δ13C values of sympatric colobines and lower than δ13C values of sympatric Theropithecus individuals (Fig. 1). The range in δ13C values among hominins at Woranso-Mille indicates that some individuals had diets dominated by C3 foods, whereas other individuals had a significant component of C4 resources in their diet. The observed variation in δ13C values among hominins at Woranso-Mille may reflect dietary differences among hominin species; however, the fragmentary nature of the sampled teeth precludes us from connecting the stable isotope results to specific hominin taxa.
The range in δ13C values of the Woranso-Mille hominins overlaps with those from older hominins, such as Ardipithecus ramidus (median −10.3‰, range −11.2‰ to −8.5‰, n = 5) (14) and Au. anamensis (median −11.2‰, −12.0‰ to −9.5‰, n = 12) (2) (Fig. 2 and Dataset S2). The distribution of δ13C values of the Woranso-Mille hominins are significantly more positive than δ13C values of Au. anamensis (P = 0.002). The δ13C values of the Woranso-Mille hominins are indistinct from the majority of younger Pliocene hominins, including Au. afarensis (1), Australopithecus africanus (4, 15–18), and Kenyanthropus platyops (2), with the exception of two individuals of Australopithecus bahrelghazali that incorporated significantly more C4 resources in their diet (19) and Australopithecus sediba, whose diet was restricted to C3 foods (20). The Woranso-Mille hominins also have substantially more C4 foods in their diets than the extant primates for which carbon isotope data from tooth enamel are available (Dataset S3), but we note that these data have mostly been generated from animals living in forests and do not represent the full spectrum of environments in Africa today. In summary, the δ13C values from the Woranso-Mille hominins indicate that C4 foods were a significant component to hominin diets as early as 3.76 Ma.
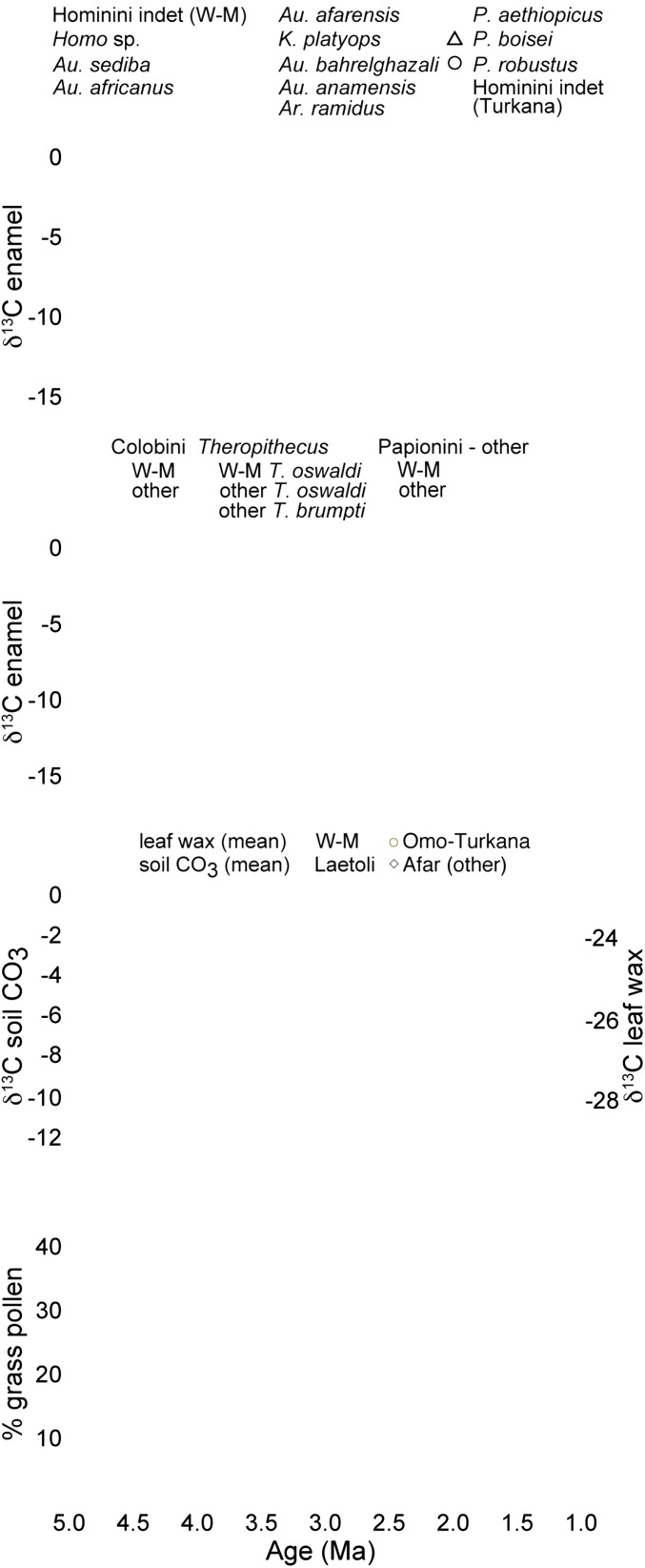
Fig. 2.
The δ13C values of African fossil hominin (A) and cercopithecid (B) tooth enamel and (C) soil carbonate and leaf wax C30-acid and (D) the percentage of grass pollen from eastern Africa. Published fossil hominin and cercopithecid δ13C data are from refs. 1, 2, 4, 14, 16–29, and 31–34. Soil carbonate δ13C values are plotted as means for 100-ky age bins to view long-term trends but are also plotted for individual soils from the Omo-Turkana, Afar, and Laetoli regions (9, 37, 41, 42, 56–65); ages are assigned as in original publication or using the assignment provided in ref. 66. Mean leaf wax δ13C values (C30 n-alkanoic acids) for 100-ky bins and percentage of grass pollen from the Deep Sea Drilling Project Site 231 are from refs. 38 and 40. Fossil ages are plotted as midpoints if the age constraint is a range of dates. In A−C, more positive δ13C values indicate an increase in the contribution of C4 vegetation. In C, error bars are 1σ on the mean.
Cercopithecids.
The δ13C values of the cercopithecids sampled from Woranso-Mille represent diets that ranged in proportions of C3 and C4 resource use (Figs. 1 and 2). The δ13C values of colobines average −11.9 ± 1.3‰ (n = 9) and represent diets of either pure C3 vegetation or with minor amounts of C4 plants and/or water-stressed C3 vegetation. The δ13C values of T. oswaldi cf. darti sampled from Woranso Mille average −3.4 ± 1.8‰ (n = 39) and represent diets that are compatible with the inclusion of a large proportion of C4 foods. The δ13C values from the other papionin samples, referred to as non-Theropithecus papionins henceforth, average −8.8 ± 3.9‰ and range from −12.0‰ to −2.6‰ (n = 9), reflective of diets that included little to no C4 vegetation for some animals and a majority of C4 vegetation for others.
Collectively, the T. oswaldi cf. darti teeth yield the highest δ13C values among all primates sampled from Woranso-Mille. They are distinct from δ13C values of the non-Theropithecus papionins, colobines, and hominins and from δ13C values of all other nonprimate taxa (P < 0.001), except for hippopotamids. The δ13C values of T. oswaldi cf. darti from Woranso-Mille are indistinguishable from the oldest Theropithecus sampled from the Turkana Basin, Theropithecus brumpti (21), but significantly lower than δ13C values from T. oswaldi that are younger than 2.0 Ma in Turkana, from Olorgesailie in Kenya, and from Swartkrans, Makapansgat, and Sterkfontein in South Africa (18, 21–24) (Fig. 2). All fossil Theropithecus δ13C values are significantly higher than the carbon isotope data available for extant primates in Africa (Dataset S3). Our data are consistent with studies from several sites in South Africa that show that Theropithecus consumed more C4 vegetation than other cercopithecids and contemporaneous hominins (Fig. 2 and Fig. S2) (18, 22–24).
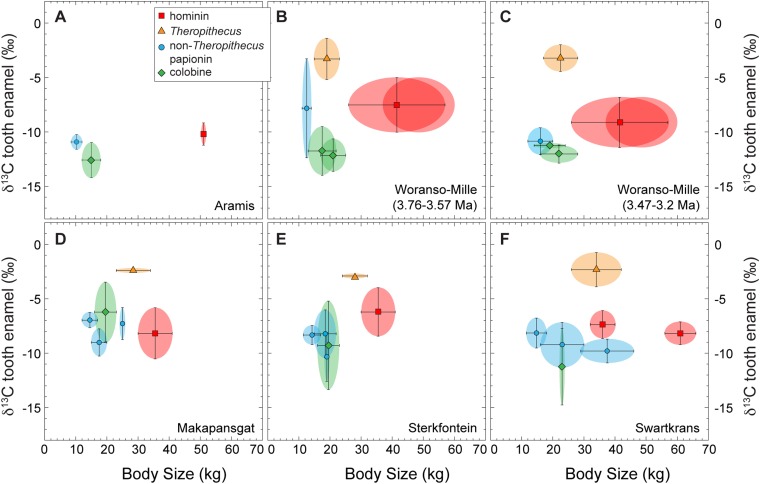
Fig. S2.
Body size vs. δ13C values of tooth enamel for fossil assemblages with isotopic data from multiple primate taxa for (A) Aramis, (B) Woranso-Mille 3.76−3.57 Ma, (C) Waranso-Mille 3.47−3.2 Ma, (D) Makapansgat, (E) Sterkfontein, and (F) Swartkrans. Size is plotted as the midpoint between the mean estimated body mass for females and males and is used here as a way to represent the range of physiologies among primates at a site. Error bars for body size represent the midpoint between male and female body mass. If error bars are missing, then data represent the only data available, which is either the mean value for males or females or an average estimate for the species. The body mass of the Woranso-Mille hominins is plotted both as the full estimated range for early Pliocene hominins and also as a more restricted range for Au. afarensis, both from ref. 73. The δ13C values are plotted as the means of the available data in reference to VPDB; error bars represent 1σ on the mean but are missing if n = 1. See Dataset S1 for the δ13C data from Woranso-Mille, Dataset S2 for the δ13C data from other sites, and Dataset S6 for body mass data.
The δ13C data from colobines come from at least two species at Woranso-Mille: Cercopithecoides cf. meaveae and one or more larger colobines. We do not have enough δ13C data from each taxon to detect differences among them, but they are all consistent with diets dominated by C3 vegetation, with δ13C values that are indistinct from the non-Theropithecus papionins and Giraffa sp. at Woranso-Mille (P > 0.1) but significantly lower than δ13C values of Theropithecus, hominins, and the nonprimate mammals at Woranso-Mille (Fig. 1). The δ13C values from colobines at Woranso-Mille are indistinct from δ13C values published from fossil colobines at other localities in Ethiopia and South Africa (Fig. 2) (14, 22, 25, 26); however, they are significantly higher than carbon isotope data from extant colobines (Dataset S3), which might indicate either a small component of C4 in their diet, a difference in digestive physiology, or that the C3 resources they consumed had higher δ13C values than the plants consumed by extant primates in tropical evergreen and rainforests.
The δ13C values of non-Theropithecus papionins at Woranso-Mille overlap with δ13C values of hominins, colobines, giraffids, and Tragelaphus but are significantly lower than those of T. oswaldi cf. darti, the non-Tragelaphus bovids, hippopotamids, N. euilus, and Proboscidea. Among the nine teeth sampled, six yield δ13C values < −9‰ (−11.4 ± 1.0‰, n = 6) and overlap with the colobine δ13C data; however, three of the teeth (all from the 3.76–3.57 Ma interval) yield distinctively higher δ13C values (−3.7 ± 1.0‰), indicating diets with significant amounts of C4 vegetation. There is nothing about the size, morphology, or anatomical position of these teeth that set them apart; as such, we interpret these data to indicate that either (i) some non-Theropithecus papionins overlapped in their dietary behavior with Theropithecus and consumed large amounts of C4 vegetation or (ii) they are early Theropithecus that used C4 resources but with tooth morphology that is not distinct enough to characterize them as Theropithecus. The δ13C values of the Woranso-Mille non-Theropithecus papionins are not significantly different from δ13C of other fossil non-Theropithecus papionins from Ethiopia, Tanzania, and South Africa (14, 18, 22–29) (Fig. 2).
Tooth Enamel Oxygen Isotope Data.
The δ18O values of teeth samples from Woranso-Mille range from −9.0‰ to +6.3‰ (Fig. 1) and exhibit fewer taxonomic distinctions than observed for δ13C. The majority of taxa have overlapping δ18O values, with the exception of the hippopotamid teeth, which are significantly lower than δ18O values of all other taxa sampled from the 3.76–3.57 Ma time interval (P < 0.02), except for Proboscidea, N. euilus, and sivatheres. We did not make this comparison for the other time intervals, as we only sampled hippopotamids from the 3.76–3.57 Ma time interval. However, among the samples from the youngest time interval (3.47–3.2 Ma), there are no distinctions in δ18O among taxa, except for δ18O of sivatheres, which are significantly higher than those of Theropithcus and N. euilus. There are not enough samples from the middle time interval (3.57–3.47 Ma) to make meaningful comparisons. Among taxa that were sampled from multiple time intervals (all of the primates, giraffids, Tragelaphus, and N. euilus), there are no temporal distinctions in the distributions of δ18O values, with the exception of Theropithecus and N. euilus, which exhibit an increase in mean δ18O values of 3.0‰ and 3.9‰, respectively, between the 3.76–3.57 Ma and 3.47–3.2 Ma sampling intervals. The total range in δ18O values among hominins (8.1‰) is similar to that for sympatric primates (6.5–7.8‰) and other mammals (5.0–11.0‰) at Woranso-Mille for which more than five teeth were sampled.
Absolute δ18O values of fossil teeth are difficult to compare between fossil localities (30); however, the low δ18O values of the hippopotamid teeth relative to most other taxa at Woranso-Mille are consistent with observations from other fossil sites in the Afar, including those with oxygen isotope data from hominins (1, 14). The offset between the giraffid and hippopotamid δ18O values in the 3.76–3.57 Ma aged sediments at Woranso-Mille is 3.3 ± 2.4‰, which can be compared with the δ18O giraffid−hippopotamid offset observed among fauna associated with Ar. ramidus at Aramis (9.0 ± 3.0‰) (14) and with Au. afarensis at Hadar (5.4 ± 4.0‰) (1). The oxygen isotope data may indicate a slightly wetter environment in Woranso-Mille at 3.76–3.57 Ma that at Hadar (ca. 3.4 Ma) or Aramis (4.4 Ma), assuming the relationship between aridity and the offset in δ18O values of giraffids and hippopotamids in eastern Africa today (30) applies to the Pliocene.
Soil Carbonates.
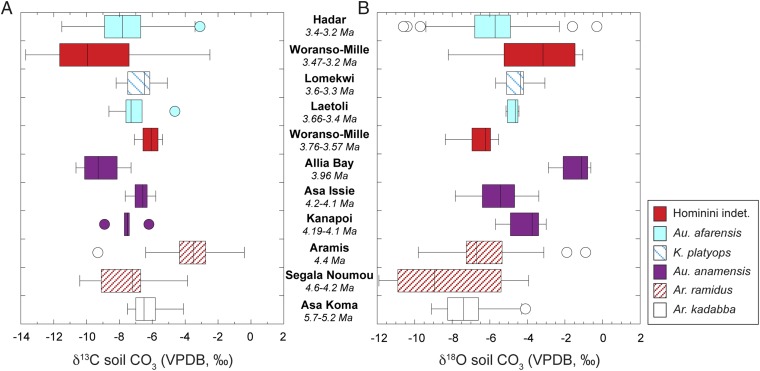
Soil carbonates from hominin fossil-bearing sequences at Woranso-Mille yield mean δ13C values of −6.1 ± 0.6‰ (n = 8) at Aralee Issie (3.76–3.57 Ma) and −9.2 ± 4.0‰ (n = 6) from Burtele and Nefuraytu (3.47–3.2 Ma) (Fig. S3 and Dataset S4). This distribution is similar to δ13C values of soil carbonates from Au. anamensis-, K. platyops-, and Au. afarensis-bearing sequences (Fig. S3 and Dataset S5). The δ18O values of soil carbonates at Woranso-Mille (−6.5 ± 0.9‰ at Aralee Issie and −3.7 ± 2.7‰ at Burtele and Nefuraytu) are within the range of δ18O values for soil carbonates from other Pliocene hominin-bearing sequences in eastern Africa (Fig. S3).
Fig. S3.
Distribution of δ13C (A) and δ18O (B) values of soil carbonates from fossil hominin-bearing strata at Worano-Mille and from other early to mid Pliocene sedimentary sequences that are associated with hominin fossils. Data from the late Miocene sediments bearing Ardipithecus kadabba fossils are also included for reference. See Datasets S4 and S5 for data and references. In these box plots, median values are marked by a vertical line within the box, the edges of the boxes represent quartile values, the horizontal lines indicate the range, and circles represent either single points (n = 1) or outlier values that are more than 1.5 times the interquartile distance.
Discussion
C4 Resource Use Among Pliocene Hominins and Cercopithecids.
The carbon isotope data from Woranso-Mille indicate that hominins included significant amounts of C4 resources in their diets as early as 3.76 Ma (Fig. 2). In this regard, δ13C values of the Woranso-Mille hominins indicate diets that are distinct from those of preceding taxa, Au. anamensis and Ar. ramidus, but similar to those of the majority of younger hominins, including Au. afarensis, K. platyops, and Au. africanus, although not to Au. bahrelghazali or Au. sediba (1, 2, 4, 14–20, 29, 31–34).
The expansion of hominin diets in the early Pliocene appears to be contemporaneous with the appearance of the first recognizable forms of Theropithecus (10, 35, 36). There are two well-known lineages of the genus, T. brumpti, largely restricted to the Turkana Basin, and the much more cosmopolitan T. oswaldi; both first appear ca. 3.8–3.6 Ma (10). The δ13C values for the earliest T. brumpti show that it incorporated a large component of C4 resources in its diet (21). The δ13C values from Woranso-Mille extend this record of early C4 consumption to the T. oswaldi lineage. The C4-dominated diets of Theropithecus are unique among the cercopithecids; colobines maintain C3-dominated diets through the Pliocene, and non-Theropithecus papionins always maintained a smaller component of C4 in their diet than sympatric populations of Theropithecus (Fig. 2, Fig. S2, and Dataset S6). It is notable that δ13C values of hominin teeth are consistently higher than δ13C values of colobines throughout the Pliocene, indicating that (i) hominin and colobine diets were always distinct, (ii) hominins and colobines had different carbon isotope diet−tissue fractionation factors, reflecting differences in their physiologies, or (iii) hominins consumed some C4 foods since 4.4 Ma.
Environmental Change.
Environmental context is an important part to understanding the evolutionary implications of the dietary expansion among hominins and Theropithecus to include C4 resources; however, it has been difficult to establish definitive links between diet and environment for fossil primates (4, 21). The incorporation of significant amounts of C4 resources in primate diets in the early Pliocene occurred amid a gradual trend of increasing C4 vegetation in Africa that began in the late Miocene (37–39) (Fig. 2). This increase in C4 vegetation is generally viewed as a decrease in woody cover (37), but pollen records indicate that C3 grasses were present throughout the Pliocene and into the Pleistocene (38, 40). Pollen abundances and δ13C values of leaf waxes and soil carbonates indicate large proportions of grasses and C4 plants in eastern Africa in the earliest Pliocene (5.0–4.0 Ma), with decreased amounts of C4 vegetation and grasses by 4.0 Ma, followed by a small rise in C4 vegetation between 3.9 and 3.7 Ma (37, 38, 40–42), which is coincident with the expansion of primate diets to include significant amounts of C4 resources. Although the coincidence makes it tempting to link the C4 dietary expansion among hominins to environmental factors, there are reasons to be cautious: (i) the soil carbonate records are sparse from this time interval, and (ii) when δ13C values of soil carbonates from hominin-bearing strata are compared directly (Fig. S3), there is no clear connection between environmental C4 abundance and C4-dominated diets among hominins. The oxygen isotope data from fossil giraffid and hippopotamid teeth may indicate wetter environments at Woranso-Mille than at both earlier and later hominin sites, but this could reflect local variation in water availability that is typical in eastern Africa today and does not necessitate any major climate change coincident with the inclusion of significant amounts of C4 resources in hominin or Theropithecus diets. Given the collective data that are available, we do not see strong environmental trends that can be linked confidently to the C4 dietary expansion among hominins and Theropithecus.
Dietary Breadth in Primate Communities.
The combination of the expanded breadth in hominin diet and the appearance of the T. oswaldi lineage as early as 3.76 Ma marks a major change in African primate communities. The appearance of Theropithecus is important to hominin paleoecology because Theropithecus was a large fraction of the identifiable mammalian fossils at Woranso-Mille (10) and was predominant in nearly all eastern African mammalian communities from 3.5 Ma to 1.0 Ma (36). The increased range in δ13C values of both hominins and cercopithecids after 3.76 Ma indicates that primate dietary breadth increased with the appearance of Theropithecus, which ultimately became a committed grazer (21). The use of both C3 and C4 foods by hominins likely reflects that they were generalists who could thrive in different environments, eating foods of varying quality and type (1, 4, 43). The inclusion of significant amounts of C4 foods among hominins and papionins indicates niche broadening that appears unique among primates in the early Pliocene. We are not aware of significant dietary changes in other African mammalian orders at this time; Artiodactyla, Perissodactyla, and Probsocidea started to consume significant amounts of C4 resources several million years earlier, in the late Miocene (39).
Relationship Between Diet and Morphology.
The craniodental features characterizing australopiths are usually interpreted as a character complex associated with adaptations for hard-object, tougher, and more abrasive food resources (44, 45), although other interpretations such as those based on dental microstructure may not corroborate the former interpretation (46). The first appearance of these features is interpreted as an adaptation to eating a broader array of resources than hominins did previously (47, 48). However, the niche expansion indicated by dentognathic morphology at 4.2 Ma is not reflected in carbon isotope data (2, 4, 14). Intake of large amounts of C4 foods as early as 3.76 Ma may represent a subsequent dietary change, wherein hominins further increased their dietary breadth (Fig. 2). Increasing enamel thickness, megadontia, and mandibular robusticity among Pliocene hominins is consistent with the consumption of more C4 food resources, which might have included underground storage organs (i.e., rhizomes, corms) (1, 49, 50). The long-term relationships between dentognathic morphology and C4 dietary intake is exhibited by correlations between δ13C values and both postcanine and mandibular size for australopithecines (4); however, morphological changes are incremental and do not reflect the stepwise increase in C4 resource consumption. We note that microwear data from Au. anamensis and Au. afarensis teeth are similar to one another and are not consistent with morphological changes that are often associated with increased specialization for hard-object feeding; instead, they are consistent with diets of soft and tough foods (51).
Among papionins, adaptations to terrestrial behavior were already apparent by the late Miocene (52), and all evidence suggests that the earliest Theropithecus was fully terrestrial (10, 36, 53, 54). The main morphological changes that occurred in Theropithecus 3.8–1.0 Ma include increasing body size and the dentognathic specialization for grazing (36, 53, 55), but the evidence for C4-dominated diets among the earliest Theropithecus fossils indicates that the grazing behavior precedes most of the dental specializations for eating grass, although we recognize that Theropithecus’ initial use of C4 grass may have focused on the nonblade part of grass.
Conclusions
Stable carbon isotope data from Woranso-Mille show that both hominins and Theropithecus included large proportions of C4 foods in their diets as early as 3.76 Ma. This increased use of C4 foods among both hominins and Theropithecus represents an expansion of dietary niche among primates to include both C3 and C4 resources that became typical in subsequent fossil primate communities for which there are isotopic data (Fig. S2). The C4 dietary expansion was relatively late among primates, as other African mammalian orders had been consuming large amounts of C4 foods since the late Miocene (39). For Theropithecus, the reliance on C4 foods represents the initiation of its dietary specialization and its role as an abundant and widespread grazer within African mammalian communities (13). The evidence for C4-dominated diets among the earliest identified specimens of Theropithecus precedes morphological specializations for grazing and provides a clear example of behavioral change preceding morphological adaptation. For hominins, the isotopic evidence for dietary expansion postdates the beginning of major morphological dentognathic changes among australopiths (47, 48) and, as such, it represents a subsequent phase of increasing dietary breadth. The unequivocal incorporation of C4 resources by hominins and cercopithecids as early as 3.76 Ma demonstrates that primates were expanding the types of food resources and potentially the environments that they were able to exploit.
Methods
Fossil teeth were sampled in the National Museum of Ethiopia in Addis Ababa. Teeth were cleaned with gentle abrasion before sampling. Hominin teeth were sampled along previously broken surfaces. Enamel powders were treated with 10% (vol/vol) H2O2 and 0.1 M buffered acetic acid solution for 15 min each, rinsed with deionized water three times after each treatment, and dried at 60 °C overnight before analysis. Soil carbonates were sampled from distinct pedogenic carbonate zones ≥ 50 cm below the contact with the overlying unit. After digestion in phosphoric acid, 13C/12C and 18O/16O ratios of enamel and carbonate powders were measured on a Thermo MAT 253 isotope ratio mass spectrometer at Johns Hopkins University. External precision (1σ) of δ13C and δ18O values of the working fossil tooth enamel standards over the course of analysis was 0.3‰ and 0.2‰ for δ13C and δ18O, respectively. Isotope data are reported relative to the Vienna Pee Dee Belemnite standard. Statistics were performed in Kaleidograph using an unpaired Wilcoxon−Mann−Whitney Rank Sum Test.
SI Methods
Geologic Context and Age Constraints for Woranso-Mille Fossils.
Although individual fossils can be placed in more tightly constrained time intervals, for the purposes of this study, fossils were placed in three stratigraphic groupings. The oldest interval is bound by two radiometrically dated tephra, the Mille tuff (3.76 ± 0.02 Ma) and the Kilaytoli tuff (KT, 3.57 ± 0.014 Ma). The middle interval is bound by the KT at the base and the Burtele Tuff (3.469 ± 0.008 Ma) at its top (7, 9, 67). The youngest time interval is bound by the Burtele Tuff at its base and extends to 3.2 Ma (constrained by sedimentation rates) (9). Localities in the lower age bin (3.76–3.57 Ma) include ARI-VP-1, ARI-VP-2, ARI-VP-3, ARI-VP-5, KSD-VP-1, MKM-VP-1, MSD-VP-1, MSD-VP-2, MSD-VP-3, MSD-VP-5, MSD-VP-8, and MSD-VP-9. Localities included in the middle age bin (3.57–3.47 Ma) are BRT-VP-3 and KSD-VP-1. Localities included in the upper, younger age bin (3.47–3.2 Ma) are BKI-VP-2, BRT-VP-1, BRT-VP-2, LAD-VP-1, LAD-VP-2, LDD-VP-1, and NFR-VP-1. Note that specimens from KSD-VP-1 are included in either the middle or lower age bin depending on their position relative to a fault that cuts through this locality.
Sampling and Analytical Methods.
Fossil teeth and soil carbonates were sampled as described in Methods. To avoid sampling multiple teeth from one individual, we targeted the third molar where possible and did not sample teeth from the same taxonomic group if the fossils were recovered in close proximity to one another. Tooth enamel and soil carbonate powders were reacted with 100% H3PO4 under vacuum at 90 °C using a common acid bath device described by ref. 68. The resultant, purified CO2 was then transferred to the microvolume of dual inlet system on a Thermo MAT 253 isotope ratio mass spectrometer at Johns Hopkins University for determination of 13C/12C and 18O/16O ratios. CO2 was measured in reference to a working reference gas calibrated during each run to NBS-19 and calcite and bioapatite working reference materials. The CO2 yields of enamel samples indicate that they contained 4–9% carbonate and did not contain large amounts of exogenous carbonate. An acid fractionation factor of 1.00725 (90 °C) was used for δ18O. All isotope data are reported relative to the Vienna Pee Dee Belemnite (VPDB) standard. Results are presented in Datasets S1 and S4.
Interpretation of δ13C Values.
We discuss carbon isotopic values using δ-notation in per mil (‰) units, where δ13C = (Rsample/Rreference – 1) × 1,000, and R represents the 13C/12C ratio of sample or reference material. For the interpretation of enamel δ13C values, we assign nominal preindustrial δ13C plant values of −26‰ and −12‰ to −10‰ for C3 and C4 plants in eastern Africa and assume that the isotope enrichment between diet and enamel for primates is ca. 14‰ (5), such that an animal with a pure C3 diet would have an enamel δ13C value of ca. −12‰ and an animal with a pure C4 diet would have an enamel δ13C value of ca. +2‰ to +4‰. We use these values only as rough guides to estimating the proportions of C3 and C4 resources consumed by an animal over the course of enamel mineralization, because there is a range in δ13C values of vegetation (diet) and there is uncertainty in the carbon isotope enrichment between diet and bioapatite for primates (5, 69). This is the approach used by ref. 2 to estimate the proportion of C3 and C4 plants in hominin diets. Wynn et al. in ref. 1 take a different approach and assign end-member δ13C values of enamel representing animals that had diets composed entirely of either C3 or C4 resources based on median values for giraffes and Alcelaphines, respectively. There were not sufficient numbers of fossil teeth representing Alcelaphines at the localities we sampled to take this approach with the Woranso-Mille data.
When we compare tooth enamel δ13C values from fossils to those of extant animals, all of the data from extant animals are corrected to δ13C values of CO2 in A.D. 1750 values as done by ref. 2, using data from ref. 70 for pre-1980 δ13CCO2-atm values and data from Mauna Loa, reported by ref. 71, for δ13CCO2-atm after 1980, assuming that δ13C value of atmospheric CO2 was −6.3‰ in 1750 A.D. These values are indicated in the text, Fig. 2, and Dataset S3 as δ13C1750. As noted by ref. 2, these values provide a maximum correction because tooth enamel formed before the death of the animal.
Supplementary Material
Acknowledgments
We thank Benjamin Kahn for assistance in compiling data, Sophie B. Lehmann for help with the isotopic analyses, and Daryl Codron for access to data. We thank the Authority for Research and Conservation of Cultural Heritage and the National Museum of Ethiopia of the Ministry of Culture and Tourism for permission to conduct the field and laboratory components of this research. We thank the Afar Regional State and the people of the Woranso-Mille area for their support of this research. We are grateful for the comments on earlier versions of this manuscript by three anonymous reviewers. This work was supported by the National Science Foundation (BCS-1125345, BCS-1124075, BCS-1124713, BCS-1124716, and BCS-1125157), Johns Hopkins University, and the Cleveland Museum of Natural History.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 12232.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424982112/-/DCSupplemental.
References
- 1.Wynn JG, et al. Diet of Australopithecus afarensis from the Pliocene Hadar Formation, Ethiopia. Proc Natl Acad Sci USA. 2013;110(26):10495–10500. doi: 10.1073/pnas.1222559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110(26):10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sage RF, Monson RK. C4 Plant Biology. Academic; San Diego, CA: 1999. [Google Scholar]
- 4.Sponheimer M, et al. Isotopic evidence of early hominin diets. Proc Natl Acad Sci USA. 2013;110(26):10513–10518. [Google Scholar]
- 5.Cerling TE, Harris JM. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120(3):347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 6.Haile-Selassie Y, Deino A, Saylor B, Umer M, Latimer B. Preliminary geology and paleontology of new hominid-bearing Pliocene localities in the central Afar region of Ethiopia. Anthropol Sci. 2007;115(3):215–222. [Google Scholar]
- 7.Haile-Selassie Y, et al. An early Australopithecus afarensis postcranium from Woranso-Mille, Ethiopia. Proc Natl Acad Sci USA. 2010;107(27):12121–12126. doi: 10.1073/pnas.1004527107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haile-Selassie Y, Saylor BZ, Deino A, Alene M, Latimer BM. New hominid fossils from Woranso-Mille (Central Afar, Ethiopia) and taxonomy of early Australopithecus. Am J Phys Anthropol. 2010;141(3):406–417. doi: 10.1002/ajpa.21159. [DOI] [PubMed] [Google Scholar]
- 9.Haile-Selassie Y, et al. A new hominin foot from Ethiopia shows multiple Pliocene bipedal adaptations. Nature. 2012;483(7391):565–569. doi: 10.1038/nature10922. [DOI] [PubMed] [Google Scholar]
- 10.Frost SR, Jablonski NG, Haile-Selassie Y. Early Pliocene Cercopithecidae from Woranso-Mille (Central Afar, Ethiopia) and the origins of the Theropithecus oswaldi lineage. J Hum Evol. 2014;76:39–53. doi: 10.1016/j.jhevol.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Geraads D, Melillo S, Haile-Selassie Y. Middle Pliocene Bovidae from Hominid-bearing sites in the Woranso-Mille area, Afar region, Ethiopia. Palaeont. Afr. 2009;44:59–70. [Google Scholar]
- 12.Haile-Selassie Y, et al. New species from Ethiopia further expands Middle Pliocene hominin diversity. Nature. 2015;521(7553):483–488. doi: 10.1038/nature14448. [DOI] [PubMed] [Google Scholar]
- 13.Jablonski NG, Frost SR. Cercopithecoidea. In: Werdelin L, Sanders WJ, editors. The Cenozoic Mammals of Africa. Univ Calif Press; Oakland: 2010. pp. 393–428. [Google Scholar]
- 14.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326(5949):87–93. [PubMed] [Google Scholar]
- 15.Lee-Thorp JA, Sponheimer M, Passey BH, de Ruiter DJ, Cerling TE. Stable isotopes in fossil hominin tooth enamel suggest a fundamental dietary shift in the Pliocene. Philos Trans R Soc Lond B Biol Sci. 2010;365(1556):3389–3396. doi: 10.1098/rstb.2010.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283(5400):368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 17.Sponheimer M, et al. Hominins, sedges, and termites: New carbon isotope data from the Sterkfontein valley and Kruger National Park. J Hum Evol. 2005;48(3):301–312. doi: 10.1016/j.jhevol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 18.van der Merwe NJ, Thackeray JF, Lee-Thorp JA, Luyt J. The carbon isotope ecology and diet of Australopithecus africanus at Sterkfontein, South Africa. J Hum Evol. 2003;44(5):581–597. doi: 10.1016/s0047-2484(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 19.Lee-Thorp J, et al. Isotopic evidence for an early shift to C₄ resources by Pliocene hominins in Chad. Proc Natl Acad Sci USA. 2012;109(50):20369–20372. doi: 10.1073/pnas.1204209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry AG, et al. The diet of Australopithecus sediba. Nature. 2012;487(7405):90–93. doi: 10.1038/nature11185. [DOI] [PubMed] [Google Scholar]
- 21.Cerling TE, Chritz KL, Jablonski NG, Leakey MG, Manthi FK. Diet of Theropithecus from 4 to 1 Ma in Kenya. Proc Natl Acad Sci USA. 2013;110(26):10507–10512. doi: 10.1073/pnas.1222571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Codron D, et al. Utilization of savanna-based resources by Plio-Pleistocene baboons. S Afr J Sci. 2005;101(5-6):245–248. [Google Scholar]
- 23.Fourie NH, Lee-Thorp JA, Ackermann RR. Biogeochemical and craniometric investigation of dietary ecology, niche separation, and taxonomy of Plio-Pleistocene cercopithecoids from the Makapansgat Limeworks. Am J Phys Anthropol. 2008;135(2):121–135. doi: 10.1002/ajpa.20713. [DOI] [PubMed] [Google Scholar]
- 24.Lee-Thorp JA, van der Merwe NJ, Brain CK. Isotopic evidence for dietary differences betweeen two extinct baboon species from Swartkrans. J Hum Evol. 1989;18(3):183–190. [Google Scholar]
- 25.Adams JW, Kegley ADT, Krigbaum J. New faunal stable carbon isotope data from the Haasgat HGD assemblage, South Africa, including the first reported values for Papio angusticeps and Cercopithecoides haasgati. J Hum Evol. 2013;64(6):693–698. doi: 10.1016/j.jhevol.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Levin NE, Simpson SW, Quade J, Cerling TE, Frost SR. Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. In: Quade J, Wynn JG, editors. The Geology of Early Humans in the Horn of Africa. Vol 446. Geol Soc Am; Boulder, CO: 2008. pp. 215–234. [Google Scholar]
- 27.Kingston JD. 2011. Stable isotopic analyses of Laetoli fossil herbivores. Geology, Geochronology, Paleoecology and Paleoenvironment, Paleontology and Geology of Laetoli: Human Evolution in Context, ed Harrison T (Springer, New York), Vol 1, pp 293−328.
- 28.Lee-Thorp JA, van der Merwe NJ. Stable carbon isotope studies of Swartkrans fossils. In: Brain CK, editor. Swartkrans: A Cave’s Chronicle of Early Man. Vol 8. Transvaal Museum; Pretoria, South Africa: 1993. pp. 251–256. [Google Scholar]
- 29.Lee-Thorp JA, van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27(4):361–372. [Google Scholar]
- 30.Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR. A stable isotope aridity index for terrestrial environments. Proc Natl Acad Sci USA. 2006;103(30):11201–11205. doi: 10.1073/pnas.0604719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee-Thorp J, Thackeray JF, van der Merwe N. The hunters and the hunted revisited. J Hum Evol. 2000;39(6):565–576. doi: 10.1006/jhev.2000.0436. [DOI] [PubMed] [Google Scholar]
- 33.Sponheimer M, et al. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science. 2006;314(5801):980–982. doi: 10.1126/science.1133827. [DOI] [PubMed] [Google Scholar]
- 34.van der Merwe NJ, Masao FT, Bamford MK. Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. S Afr J Sci. 2008;104:153–155. [Google Scholar]
- 35.Harris JM, Leakey MG, Cerling TE. Early Pliocene tetrapod remains from Kanapoi, Lake Turkana Basin, Kenya. In: Harris JM, Leakey MG, editors. Geology and Vertebrate Paleontology of the Early Pliocene Site of Kanapoi, Northern Kenya, Contributions in Science. Vol 498. Nat Hist Mus Los Angeles County; Los Angeles: 2003. pp. 39–113. [Google Scholar]
- 36.Frost SR. African Pliocene and Pleistocene cercopithecid evolution and global climate change. In: Bobe R, Alemseged Z, Behrensmeyer AK, editors. Hominin Environments in the East African Pliocene: An Assessment of the Faunal Evidence. Springer; Dordrecht, The Netherlands: 2007. pp. 51–76. [Google Scholar]
- 37.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 38.Feakins SJ, et al. Northeast African vegetation change over 12 m.y. Geology. 2013;41(3):295–298. [Google Scholar]
- 39.Uno KT, et al. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proc Natl Acad Sci USA. 2011;108(16):6509–6514. doi: 10.1073/pnas.1018435108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnefille R. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global Planet Change. 2010;72(4):390–411. [Google Scholar]
- 41.Cerling TE. Development of grasslands and savannas in East Africa during the Neogene. Palaeogeogr Palaeoclimatol Palaeoecol. 1992;97(3):241–247. [Google Scholar]
- 42.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. Paleosol carbonates from the Omo Group: Isotopic records of local and regional environmental change in East Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;307(1-4):75–89. [Google Scholar]
- 43.Codron D, Lee-Thorp JA, Sponheimer M, de Ruiter DJ, Codron J. What insights can baboon feeding ecology provide for early hominin niche differentiation? Int J Primatol. 2008;29(3):757–772. [Google Scholar]
- 44.McHenry HM. Relative cheek-tooth size in Australopithecus. Am J Phys Anthropol. 1984;64(3):297–306. doi: 10.1002/ajpa.1330640312. [DOI] [PubMed] [Google Scholar]
- 45.Teaford MF, Ungar PS. Diet and the evolution of the earliest human ancestors. Proc Natl Acad Sci USA. 2000;97(25):13506–13511. doi: 10.1073/pnas.260368897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macho GA, Shimizu D, Jiang Y, Spears IR. Australopithecus anamensis: A finite-element approach to studying the functional adaptations of extinct hominins. Anat Rec A Discov Mol Cell Evol Biol. 2005;283(2):310–318. doi: 10.1002/ar.a.20175. [DOI] [PubMed] [Google Scholar]
- 47.Ward CV, Leakey MG, Walker A. Morphology of Australopithecus anamensis from Kanapoi and Allia Bay, Kenya. J Hum Evol. 2001;41(4):255–368. doi: 10.1006/jhev.2001.0507. [DOI] [PubMed] [Google Scholar]
- 48.White TD, et al. Asa Issie, Aramis and the origin of Australopithecus. Nature. 2006;440(7086):883–889. doi: 10.1038/nature04629. [DOI] [PubMed] [Google Scholar]
- 49.Dominy N, Vogel E, Yeakel J, Constantino P, Lucas P. Mechanical properties of plant underground storage organs and implications for dietary models of early hominins. Evol Biol. 2008;35(3):159–175. [Google Scholar]
- 50.Peters CR, Vogel JC. Africa’s wild C4 plant foods and possible early hominid diets. J Hum Evol. 2005;48(3):219–236. doi: 10.1016/j.jhevol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Ungar PS, Scott RS, Grine FE, Teaford MF. Molar microwear textures and the diets of Australopithecus anamensis and Australopithecus afarensis. Philos Trans R Soc Lond B Biol Sci. 2010;365(1556):3345–3354. doi: 10.1098/rstb.2010.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbert CC, Goble ED, Hill A. Miocene Cercopithecoidea from the Tugen Hills, Kenya. J Hum Evol. 2010;59(5):465–483. doi: 10.1016/j.jhevol.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Frost SR, Delson E. Fossil Cercopithecidae from the Hadar Formation and surrounding areas of the Afar Depression, Ethiopia. J Hum Evol. 2002;43(5):687–748. doi: 10.1006/jhev.2002.0603. [DOI] [PubMed] [Google Scholar]
- 54.Jablonski NG, Leakey MG, Kiarie C, Antón M. A new skeleton of Theropithecus brumpti (Primates: Cercopithecidae) from Lomekwi, West Turkana, Kenya. J Hum Evol. 2002;43(6):887–923. doi: 10.1006/jhev.2002.0607. [DOI] [PubMed] [Google Scholar]
- 55.Delson E, Terranova CJ, Sargis EJ, Jablonski NG, Dechow PC. Body mass in Cercopithecidae (Primates, Mammalia): Estimation and scaling in extinct and extant taxa. Am Mus Nat Hist Anthropol Pap. 2000;83:1–159. [Google Scholar]
- 56.Cerling TE, Bowman JR, O’Neil JR. An isotopic study of a fluvial-lacustrine sequence: The Plio-Pleistocene Koobi Fora sequence, East Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 1988;63(4):335–356. [Google Scholar]
- 57.Wynn JG. Paleosols, stable carbon isotopes, and paleoenvironmental interpretation of Kanapoi, Northern Kenya. J Hum Evol. 2000;39(4):411–432. doi: 10.1006/jhev.2000.0431. [DOI] [PubMed] [Google Scholar]
- 58.Cerling TE, Harris JM, Leakey MG. Isotope paleoecology of the Nawata and Nachukui Formations at Lothagam, Turkana Basin, Kenya. In: Leakey MG, Harris JM, editors. Lothagam: The Dawn of Humanity in Eastern Africa. Columbia Univ Press; New York: 2003. pp. 605–614. [Google Scholar]
- 59.Levin NE, Quade J, Simpson SW, Semaw S, Rogers MJ. Isotopic evidence for Plio-Pleistocene environmental change at Gona, Ethiopia. Earth Planet Sci Lett. 2004;219(1-2):93–110. [Google Scholar]
- 60.Quade J, et al. Paleoenvironments of the earliest stone toolmakers, Gona, Ethiopia. Geol Soc Am Bull. 2004;116(11-12):1529–1544. [Google Scholar]
- 61.Wynn JG. Influence of Plio-Pleistocene aridification on human evolution: Evidence from paleosols of the Turkana Basin, Kenya. Am J Phys Anthropol. 2004;123(2):106–118. doi: 10.1002/ajpa.10317. [DOI] [PubMed] [Google Scholar]
- 62.Quinn RL, Lepre CJ, Wright JD, Feibel CS. Paleogeographic variations of pedogenic carbonate δ13C values from Koobi Fora, Kenya: Implications for floral compositions of Plio-Pleistocene hominin environments. J Hum Evol. 2007;53(5):560–573. doi: 10.1016/j.jhevol.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Aronson JL, Hailemichael M, Savin SM. Hominid environments at Hadar from paleosol studies in a framework of Ethiopian climate change. J Hum Evol. 2008;55(4):532–550. doi: 10.1016/j.jhevol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 64.WoldeGabriel G, Gilbert WH, Hart WK, Renne PR, Ambrose SH. Geology and geochronology. In: Gilbert WH, Asfaw B, editors. Homo erectus: Pleistocene Evidence from the Middle Awash, Ethiopia. Univ Calif Press; Berkeley: 2008. pp. 13–43. [Google Scholar]
- 65.WoldeGabriel G, et al. The geological, isotopic, botanical, invertebrate, and lower vertebrate surroundings of Ardipithecus ramidus. Science. 2009;326(5949):e1–e5. doi: 10.1126/science.1175817. [DOI] [PubMed] [Google Scholar]
- 66.Levin NE. 2013. Compilation of East Africa Soil Carbonate Stable Isotope Data. Available at dx.doi.org/10.1594/IEDA/100231. Accessed June 20, 2014.
- 67.Deino AL, et al. 40Ar/39Ar dating, paleomagnetism, and tephrochemistry of Pliocene strata of the hominid-bearing Woranso-Mille area, west-central Afar Rift, Ethiopia. J Hum Evol. 2010;58(2):111–126. doi: 10.1016/j.jhevol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Passey BH, Levin NE, Cerling TE, Brown FH, Eiler JM. High-temperature environments of human evolution in East Africa based on bond ordering in paleosol carbonates. Proc Natl Acad Sci USA. 2010;107(25):11245–11249. doi: 10.1073/pnas.1001824107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Passey BH, et al. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. J Archaeol Sci. 2005;32(10):1459–1470. [Google Scholar]
- 70.Francey RJ, et al. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B. 1999;51(2):170–193. [Google Scholar]
- 71.Keeling RF, Piper SC, Bollenbacher AF, Walker SJ. Trends: A Compendium of Data on Global Change. Carbon Dioxide Inf Anal Cent; Oak Ridge, TN: 2010. Monthly atmospheric 13C/12C isotopic ratios for 11 SIO stations. [Google Scholar]
- 72.Stöckli R, Vermote E, Saleous N, Simmon R, Herring D. The Blue Marble Next Generation - A True Color Earth Dataset Including Seasonal Dynamics from MODIS. NASA Earth Observatory; Greenbelt, MD: 2005. [Google Scholar]
- 73.McHenry HM. Body size and proportions in early hominids. Am J Phys Anthropol. 1992;87(4):407–431. doi: 10.1002/ajpa.1330870404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.