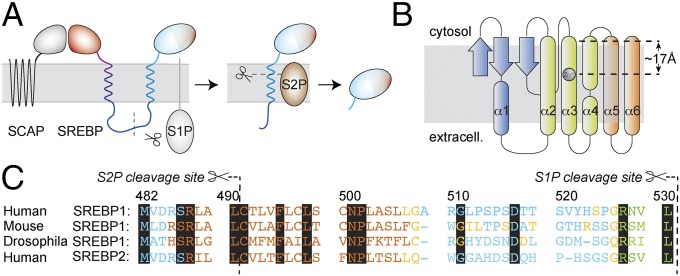
Fig. 1.
The proteolytic cascade for maturation of SREBPs. (A) After COPII vesicle transport to the Golgi, the C-terminal part of the SREBP precursor, attached to SCAP, is shed by S1P. The active transcription factor (light blue) is then liberated by RIP of the single-TM S2P substrate. (B) S2P topology derived from Protein Data Bank ID code 3B4R (13). (C) Alignment of full-length SREBP anchor sequences. Key residues, which are conserved among different kingdoms and in between SREBP-1 and -2, are shown on black background. JPRED prediction (40) of protein TM regions (brown, helix and less than 25% accessible to solvent; blue, random coil and more than 25% solvent accessible; yellow, coil and less than 25% accessible; green, β-strand and less than 25% accessible). The region predicted as a β-strand obtains random-coil propensity when only the strip until the S1P cleavage site is subjected to the prediction (SI Appendix).