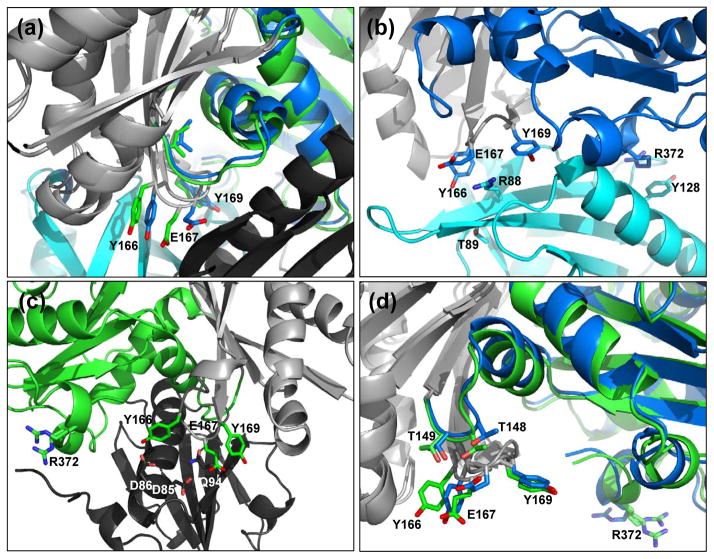
Figure 5.
Actin W-loop conformations in the wide-open and nonexchanging-closed states. (a) W-loop residues for the wide-open state (blue) and the nonexchanging-closed state (green) rotated +45° from the standard view. Light gray denotes the superimposed regions (same as in Fig. 4) and profilin and gelsolin are colored cyan and black, respectively. (b) The W-loop of wide-open state actin interacts with profilin to stabilize the interaction. Note the stacking interactions between actin residue Arg-372 and profilin residue Tyr-128. Here, actin is in the standard view. (c) Gelsolin binds the opposite face of actin. Nonexchanging-closed state actin W-loop residues interact with gelsolin to stabilize the interaction. Here, the structure is rotated 180° from the standard view. (d) Intramolecular interactions between the actin W-loop actin residues Thr-148 and Thr-149. Here, actin is in the standard view.