Abstract
Objective:
To estimate the rates and predictors of recurrent stroke among survivors of pediatric cancer who have had a first stroke.
Methods:
The Childhood Cancer Survivor Study is a retrospective cohort study with longitudinal follow-up that enrolled 14,358 survivors (<21 years old at diagnosis; diagnosed 1970–1986; survived ≥5 years after cancer diagnosis) and followed them prospectively since 1994. We surveyed 443 survivors who reported a first stroke to identify recurrent stroke, and estimated recurrent stroke rates ≥5 years after cancer diagnosis.
Results:
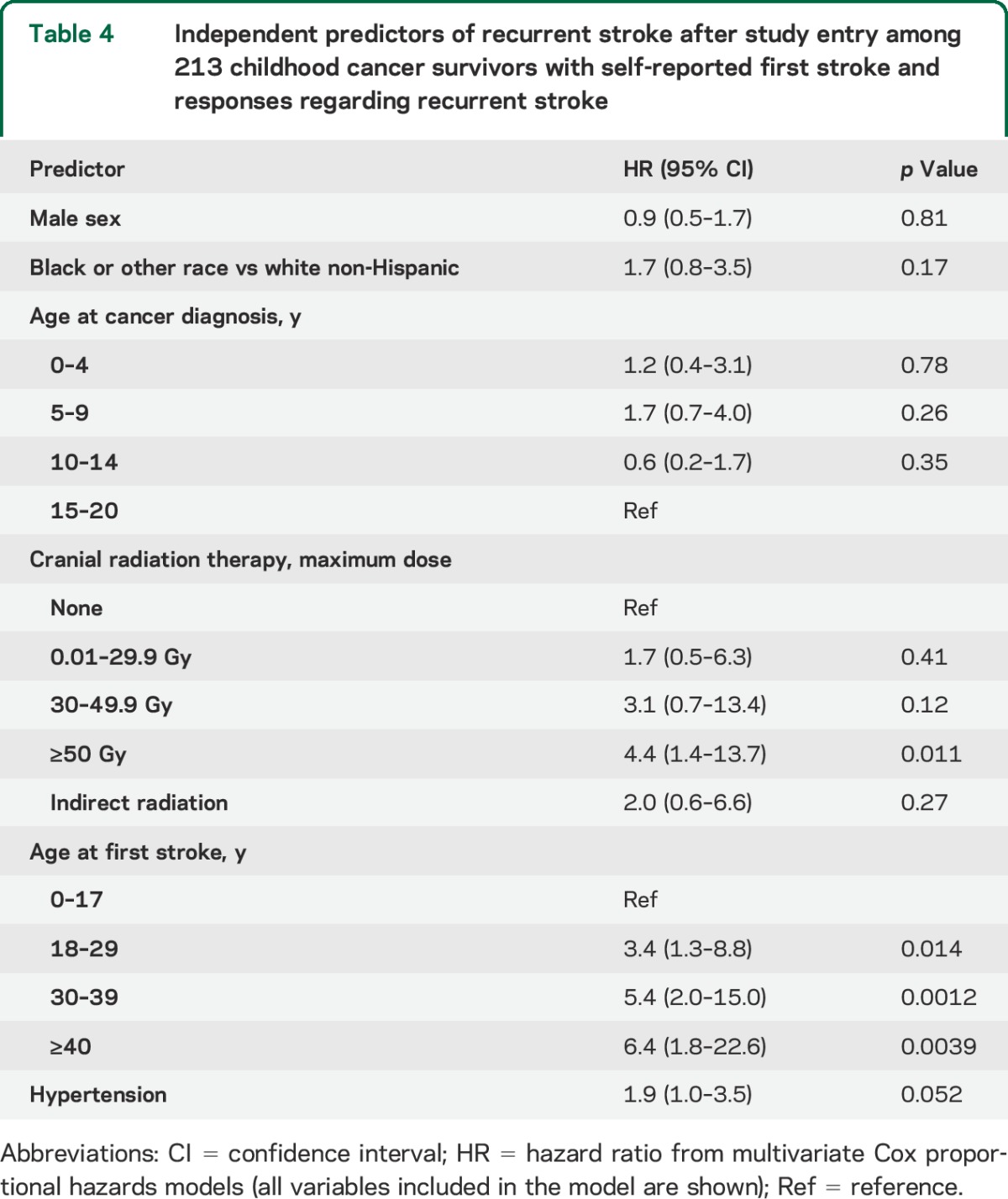
Among 329 respondents (74% response rate), 271 confirmed a first stroke at a median age of 19 years (range 0–53), and 70 reported a second stroke at a median age of 32 years (range 1–56). In a multivariable Cox proportional hazards model, independent predictors of recurrent stroke included cranial radiation therapy (CRT) dose of ≥50 Gy (vs none, hazard ratio [HR] 4.4; 95% confidence interval [CI] 1.4–13.7), hypertension (HR 1.9; 95% CI 1.0–3.5), and older age at first stroke (HR 6.4; 95% CI 1.8–23; for age ≥40 vs age 0–17 years). The 10-year cumulative incidence of late recurrent stroke was 21% (95% CI 16%–27%) overall, and 33% (95% CI 21%–44%) for those treated with ≥50 Gy of CRT.
Conclusion:
Survivors of childhood cancer, particularly those previously treated with high-dose cranial radiation, have a high risk of recurrent stroke for decades after a first stroke. Although these strokes are mostly occurring in young adulthood, hypertension, an established atherosclerotic risk factor, independently predicts recurrent stroke in this population.
Prior studies have shown that childhood cancer survivors are at increased risk of stroke but many questions remain. Recent studies suggest that stroke risk remains elevated decades after cancer treatment.1–3 Cranial radiation therapy is a particularly strong predictor of first stroke, with an apparent dose-dependent effect.1,2,4,5 The etiology of stroke in these cases is likely a radiation-induced arteriopathy, which can lead to large or small vessel infarction, although the mechanisms underlying this arteriopathy have yet to be established.6,7 In a population-based cohort of children with ischemic stroke of any etiology, those with an underlying arteriopathy had an extraordinarily high risk of recurrent stroke: 66% within 5 years of the initial stroke.8 Children with radiation-induced arteriopathy most likely fall in this high-risk group, but there are few data regarding rates and predictors of recurrent stroke in pediatric cancer survivors. A better understanding of risk factors for recurrent stroke is needed to develop strategies for secondary stroke prevention in this high-risk population.
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort study with prospective follow-up.9,10 We used a previously identified subcohort with self-reported first stroke2 and administered a new stroke survey to test the hypotheses that (1) stroke recurrence rates are high in childhood cancer survivors with first stroke and (2) cranial radiation therapy is a predictor of recurrent stroke.
METHODS
Cohort.
CCSS is an NIH-funded cohort study that originally identified 20,690 survivors of childhood cancer at 26 institutions in the United States and Canada, and recruited 14,358 of them into long-term follow-up (figure e-1 on the Neurology® Web site at Neurology.org). Eligibility criteria for enrollment are fully described elsewhere, and included age <21 years at cancer diagnosis, diagnosis between 1970 and 1986, survival for ≥5 years after diagnosis, and initial cancer treatment received at a CCSS collaborating institution.11 Extensive details regarding baseline characteristics of patients (e.g., sex and race), the cancer type, radiation doses, and therapies received were collected at the time of enrollment. Late effects of cancer and cancer therapy have been assessed prospectively through 4 follow-up surveys available for review at http://ccss.stjude.org. Vital status for participants was assessed based on linkage to the National Death Index (NDI). A detailed description of the CCSS cohort, including comparisons of survey respondents vs nonrespondents, has been published previously.9–11
Standard protocol approvals, registrations, and patient consents.
The initial CCSS study was approved by institutional review boards (IRBs) at each collaborating institution and follow-up surveys, including the stroke survey, were approved by the IRB at St. Jude Children's Research Hospital, home of the Coordinating Center.
Stroke subcohort.
CCSS patients were eligible for inclusion in the current study of recurrent stroke if they (or their proxy, if deceased) had responded either “yes” or “not sure” to questions about first stroke on any of the prior outcomes questionnaires. This cohort of patients with reported first stroke has been described recently.2 All eligible patients were contacted by mail with a new survey focused on first and recurrent stroke. If a potential patient was deceased, a modified survey was sent to his or her proxy. Those who did not respond within 30 days to the mailing were subsequently contacted by telephone. In addition, the survey was remailed to nonrespondents 5 and 9 months after the first mailing, followed by telephone calls 30 days after each mailing.
2011 stroke survey.
Patients/proxies were given the option of completing the survey by mail, online, or by telephone. The stroke survey (available at the CCSS Web site, http://ccss.stjude.org) first defined stroke using lay terminology, then asked the patient (or proxy) to confirm his or her prior report of a stroke. If confirmed, the survey asked detailed questions about the original stroke (presentation, evaluation, and treatment), and then asked if and when the patient had a recurrent stroke.
Data analysis.
We used time-to-event methodology to estimate cumulative incidence rates and predictors of stroke recurrence. Because patients were enrolled in CCSS only if they had survived 5 years after cancer diagnosis, patients are considered to have entered the study at 5 years postdiagnosis when the time period of observation for late effects began, and this defines the cohort of subjects at risk of these conditions. The questionnaires asked about stroke retrospectively, thus the survivor/proxy could have reported a first or recurrent stroke at an age prior to 5 years after diagnosis. However, to avoid survival bias, such first and recurrent strokes are summarized in descriptive tables, but not included in the time-to-event analysis. The time-to-event analyses utilized left truncation, with the period at risk for an observable recurrent stroke beginning at the time of the first stroke, or at 5 years postdiagnosis (the time point of study eligibility), whichever was later. Patients whose response to the recurrent stroke question was missing or “I don't know” were excluded from the analysis. Patients with missing age at recurrent stroke or age at recurrent stroke prior to study entry were also excluded (figure e-1).
Time from first stroke to initial recurrent stroke was based on reported integer ages, with age converted to a date equivalent to the midpoint of the year of the reported age. If the reported age at recurrent stroke was equivalent to the age at first stroke, 2 months (60 days) of follow-up was assigned, based on the median time found in other studies.8
In the absence of recurrent stroke, patients were censored at the date of the last questionnaire completed by the patient or his or her proxy, and death was treated as a competing risk event for the calculation of cumulative incidence.12 Based on cause of death from the NDI, among all CCSS patients, there were 23 deaths considered to be stroke-related. However, 17 of these patients died after the last completed questionnaire, with no indication of a stroke on a prior outcome questionnaire, so they were not included in the 2011 stroke survey. Of the remaining 6 patients, 3 had proxy responses and were included in the time to event analysis, with 2 as recurrent stroke events and one censored at death (likely death from first stroke). We calculated unadjusted hazard ratios (HRs) as a measure of relative risk using Cox proportional hazards techniques, where time to event was censored at last contact or death. To determine independent predictors of recurrent stroke, we evaluated multivariable Cox proportional hazards models,13 using univariate screening with a p value cutoff of 0.10 for inclusion in the model. Sex, race/ethnicity (white non-Hispanic vs other), and age at diagnosis were included in all models as a priori covariates. The primary predictor of interest was total dose of cranial radiation therapy, as defined and categorized for the CCSS study of predictors of first stroke.2 Other predictors included age at first stroke, cancer type, chemotherapy, diagnosis of neurofibromatosis type 1 (NF1), hypertension, smoking, and diabetes. NF1 was based on self-report on any questionnaire. Hypertension, smoking, and diabetes were treated as time-dependent variables that switched from not present to present at the earliest self-reported age at diagnosis or usage, and were defined as in our prior report.2
RESULTS
Within the overall CCSS cohort of 14,358 childhood cancer survivors followed for a mean of 23 years postdiagnosis, a total of 292 had reported a first stroke after study entry by the time of the third follow-up CCSS survey. An additional 172 patients reported a stroke prior to study entry (i.e., within the first 5 years after cancer diagnosis), and 25 indicated “not sure” on the third survey. Of these 489 patients with possible first stroke, questionnaires were mailed to 334 live patients and 109 proxies of deceased patients; 329 responses were received (74% response rate; figure e-1). Key characteristics of subjects such as sex, race, age at cancer diagnosis, age at first stroke, age at last follow-up, and radiation dose did not differ significantly between those who responded to the survey compared to those who did not (χ2 test; see table e-1).
First stroke in childhood cancer survivors.
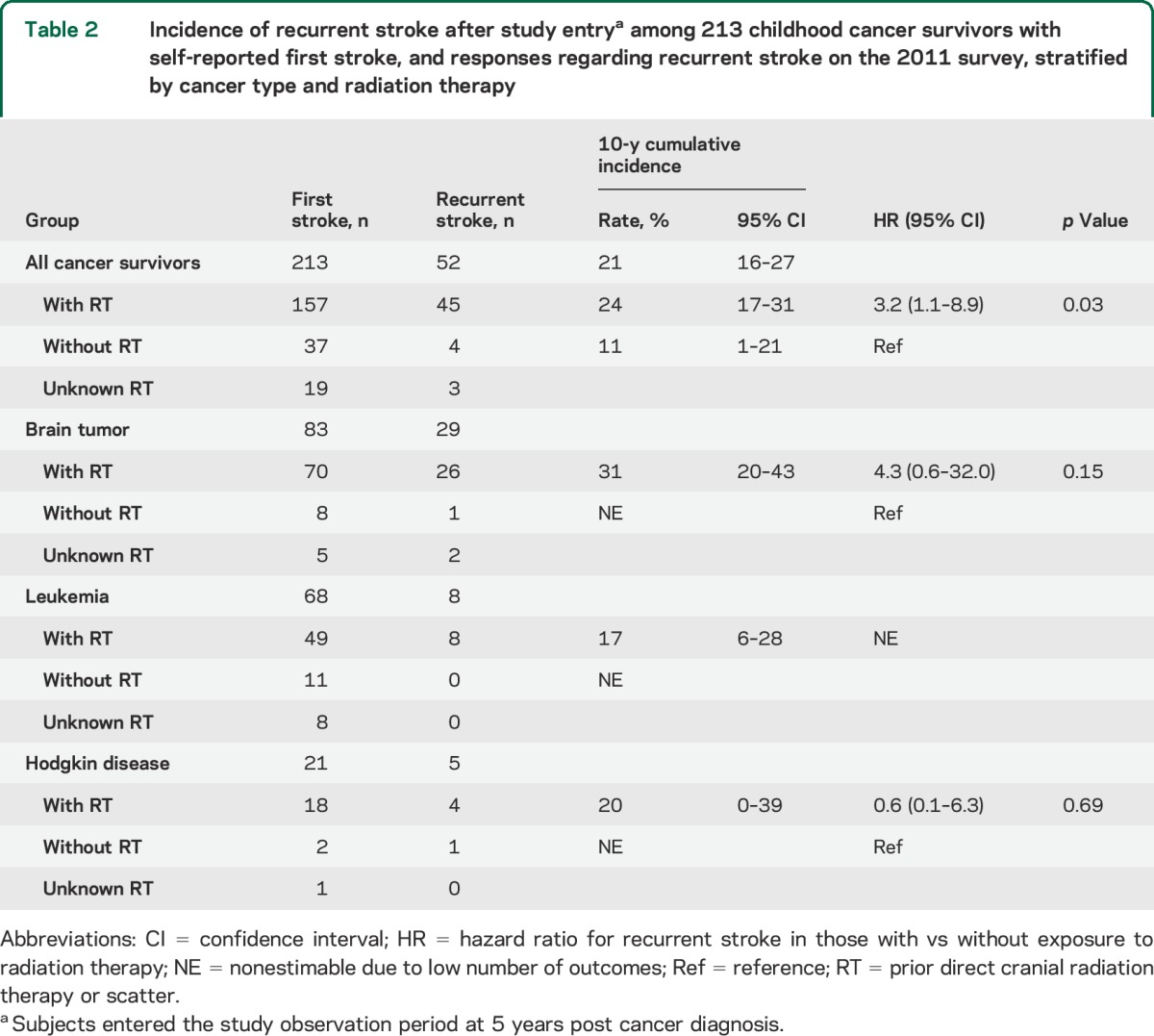
Among the respondents, 271 confirmed a first stroke. The median time from cancer diagnosis to first stroke was 10 years (interquartile range [IQR] 21 years). The most common underlying cancer types were brain tumors (44%) and leukemia (28%); the majority had received cranial radiation, although 22% of the stroke cohort had received neither cranial nor neck radiation (table e-2). The median age at first stroke was 19 years (table e-2). The most common presenting features were unilateral weakness (64%) and difficulty walking (63%) and speaking (60%); a minority of respondents (37%) reported complete recovery from the first stroke. Among 79 patients with a self-reported ischemic stroke, 51 (65%) reported poststroke treatment with an antithrombotic agent (antiplatelet or anticoagulant, in hospital or at home), as well as 13 (20%) of 65 patients with a self-reported hemorrhagic stroke.
Recurrent stroke in childhood cancer survivors.
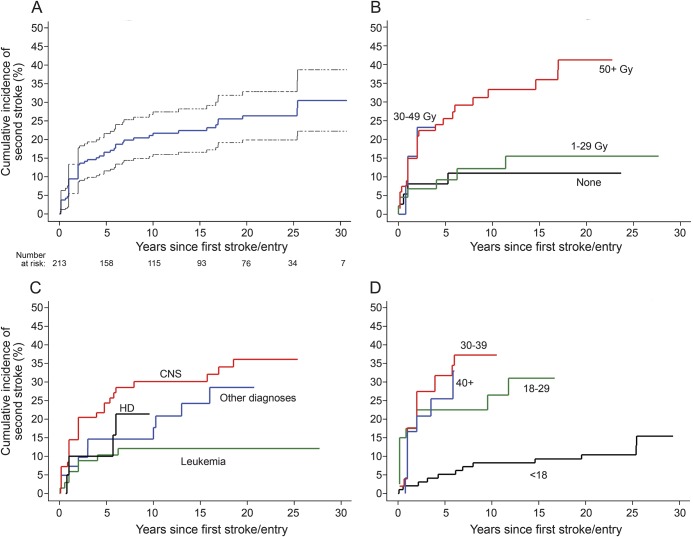
Of the 271 patients with first stroke, 70 reported a second stroke, 164 reported no recurrence, and 37 were unsure whether there was a recurrence or skipped the question. The median age at second stroke was 32 years (table 1); the median time from cancer diagnosis to second stroke was 23 years (IQR 19 years). The most common presenting features were difficulty speaking (64%) and walking (61%) and unilateral weakness (47%); only 27% reported a complete recovery from the second stroke. Among the 271 patients with first stroke, 58 could not be included in the time-to-event analysis (figure e-1); of the remaining 213, 52 reported a second stroke. The 10-year cumulative incidence of second stroke was 21% (95% confidence interval [CI] 16–27) overall (figure 1A), 33% (95% CI 21–44) for those treated with ≥50 Gy of cranial radiation, and 11% (95% CI 0%–21%) for those who received no radiation (table 2).
Table 1.
Characteristics of second stroke in 70 childhood cancer survivors reporting a recurrent stroke in the 2011 stroke survey
Figure 1. Cumulative incidence of recurrent stroke in childhood cancer survivors.
Cumulative incidence of recurrent stroke after study entry among 213 childhood cancer survivors with self-reported first stroke and responses regarding recurrent stroke on the 2011 stroke questionnaire: unstratified with 95% confidence interval (A), and stratified by maximum dose of cranial radiation therapy to any brain segment (B), cancer type (C), and age in years at first stroke (D). Curves begin at the time of first stroke or, if first stroke occurred prior to study entry, at study entry. Curves are displayed out to time point where fewer than 10 survivors were at risk. CNS = central nervous system tumors; HD = Hodgkin disease.
Table 2.
Incidence of recurrent stroke after study entrya among 213 childhood cancer survivors with self-reported first stroke, and responses regarding recurrent stroke on the 2011 survey, stratified by cancer type and radiation therapy
Predictors of recurrent stroke in childhood cancer survivors.
Stratified cumulative incidence curves suggested that cranial radiation dose, cancer type, and age at first stroke modified recurrent stroke risk (figure 1, B–D); total cranial radiation dose of ≥50 Gy, brain tumors, and age >17 years at first stroke were significant predictors of recurrence on univariate analysis (table 3). Hypertension (reported at any point prior to the second stroke or loss to follow-up) was also predictive in a univariate analysis. These remained independent predictors of recurrence on multivariate analysis (table 4); cancer type was not included in the final model due to collinearity with cranial radiation dose, and history of NF1 could not be included since only 3 patients reported a positive history.
Table 3.
Univariate predictors of recurrent stroke after study entry among 213 childhood cancer survivors with self-reported first stroke and responses regarding recurrent stroke
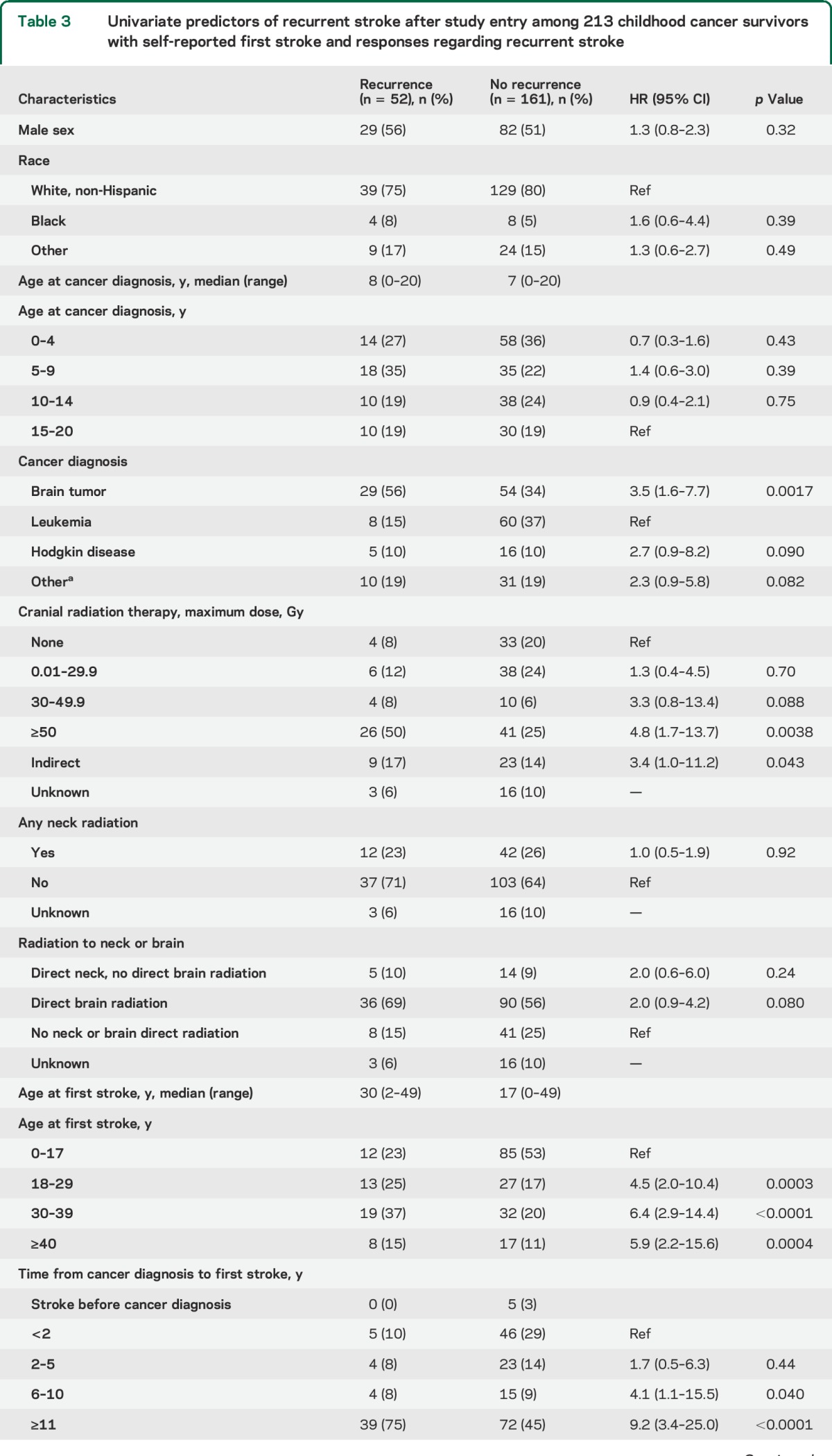
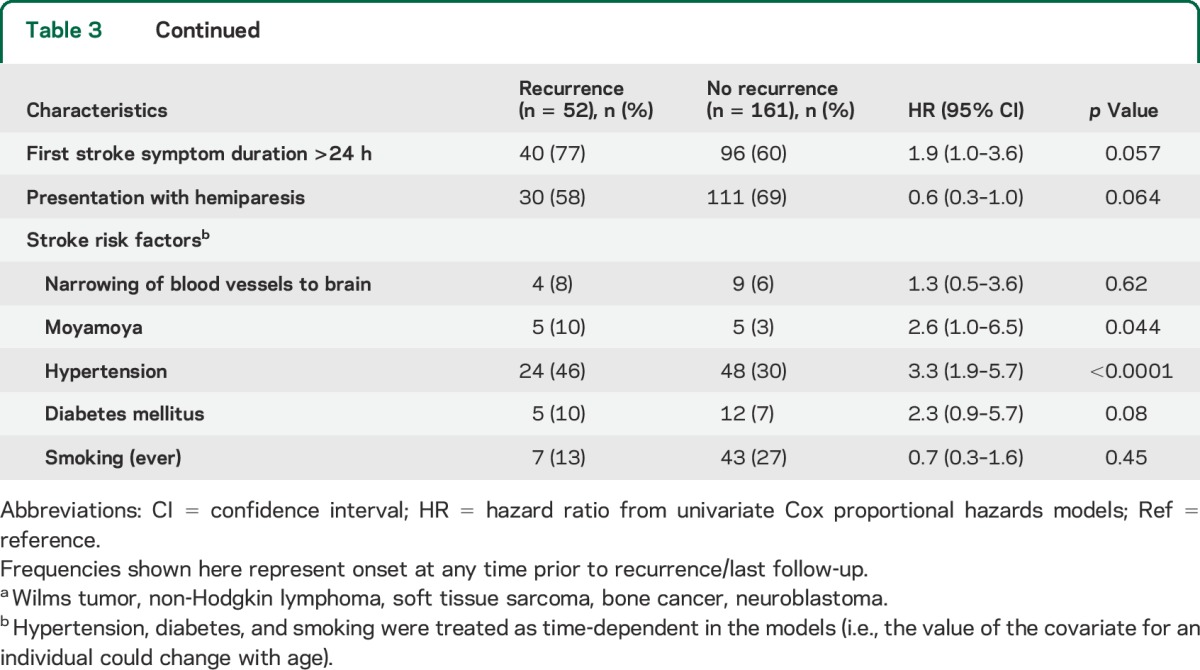
Table 4.
Independent predictors of recurrent stroke after study entry among 213 childhood cancer survivors with self-reported first stroke and responses regarding recurrent stroke
DISCUSSION
Survivors of childhood cancer with self-reported first stroke are at high risk for recurrent stroke in the decade following the first stroke. Predictors of recurrent stroke are similar to predictors of first stroke, including high-dose cranial radiation therapy and hypertension. Older age at first stroke also predicted risk of recurrence.
The cumulative rate of recurrent stroke in the general population of young adults (<50 years of age) has been estimated at approximately 10% by 10 years after a first ischemic stroke.14,15 In our cohort of childhood cancer survivors, the first strokes tended to occur in young adulthood, but the recurrent stroke rate was, overall, double that for young adults in general, with a 10-year cumulative rate of 21%. Prior cranial radiation therapy (≥50 Gy) was a significant predictor of recurrent stroke risk. Those who received no cranial radiation had a recurrent stroke risk of 11% at 10 years, similar to young adults in general; the risk tripled for those who received ≥50 Gy (33% at 10 years). Prior neck radiation was not a significant predictor of recurrent stroke risk in our study. However, our study was likely underpowered to detect an association between neck radiation and recurrent stroke.
There is one published estimate of recurrent stroke risk in this setting: a single-institution retrospective study, performed by authors of this report (H.J.F., S.M.), examined stroke risk among 19 childhood cancer survivors who had been treated with high-dose cranial or cervical radiation and reported a first stroke at any time (including those in the first 5 years after cancer diagnosis).3 Of the 19 patients with a first stroke (median age at first stroke of 24 years), 6 had recurrent strokes at a median interval of 15 months (IQR 6 months–3.2 years), yielding a cumulative 10-year recurrence estimate of 59% (95% CI 27–92). This higher point estimate, when compared with 33% (95% CI 21–44) for the CCSS stroke cohort treated with ≥50 Gy cranial radiation, may suggest underreporting of stroke in the current study. However, other methodologic differences, including differences in sample size, may also explain the apparent difference.
The mechanism underlying how cranial radiation increases stroke risk remains to be elucidated. Studies have shown that radiation therapy is associated with an occlusive disease of arteries located within or nearby the radiation field.7,16 Among children with arterial ischemic stroke of any cause, the presence of a cerebral arteriopathy, and of moyamoya in particular, has been shown to be the strongest predictor of recurrent stroke.8,17 While the development of moyamoya is a relatively early phenomenon, the elevated risk of first stroke in childhood cancer survivors appears to persist, and even increase, decades after treatment, suggesting a second mechanism for a delayed radiation arteriopathy.1,2
A prior CCSS study found that hypertension and black race—both established risk factors for atherosclerosis18,19—independently increased risk of first stroke in childhood cancer survivors.2 These findings, in addition to extensive clinical and laboratory evidence suggesting that neck radiation causes accelerated atherosclerosis of cervical vessels,7,20,21 led to a hypothesis that cranial radiation may increase stroke risk by accelerating the development of intracranial atherosclerosis. In our study, we similarly found that hypertension independently predicted risk of recurrent stroke; there was a nonsignificant trend toward an association with diabetes. Age ≥17 years at the time of first stroke also predicted recurrence, with risk increasing with each decade of advancing age. A recent analysis from the CCSS also showed that childhood cancer survivors have premature aging and early onset of cardiovascular disease.22 This lends further support to the hypothesis that early-onset atherosclerosis is on the causal pathway from cranial radiation to ischemic stroke, and suggests that earlier screening for modifiable atherosclerotic risk factors could be an avenue for improving both cardiovascular and cerebrovascular outcomes.
Strengths of this study include the relatively large size of the cohort, the long duration of follow-up, and the quality of the data on predictors such as cancer treatments. However, our findings must be considered in the context of certain limitations. It was not feasible to obtain medical records or brain imaging to confirm the reported strokes in our cohort. Hence, all strokes (first and recurrent) were measured by self-report or proxy report, which likely led to some misclassification, and also precluded reliable subclassification of stroke type (ischemic vs hemorrhagic). Subclinical or silent strokes may have gone undetected, resulting in an underestimation of total stroke frequency in the cohort. However, the 2011 stroke survey improved upon prior CCSS survey measurements of stroke outcomes by providing lay definitions of stroke before asking respondents to confirm their report of stroke, and by including questions regarding stroke presentation and treatment. Indeed, 55 previously reported first strokes were not confirmed on the stroke survey, suggesting that the lay definitions may have improved the respondents' understanding of the question. Furthermore, our high response rate to the stroke survey suggests that there was no significant participation bias (i.e., both patients with and without recurrent stroke responded to the stroke survey). Our measurements of atherosclerotic risk factors were also based on self-report, and we had to make assumptions regarding the onset of these risk factors. Also, because patients were enrolled in CCSS only if they survived 5 years post cancer diagnosis, our study is missing children who died within the first 5 years (a group that may have been at particularly high risk for first and recurrent stroke). Hence, our results are only generalizable to ≥5-year survivors of childhood cancer, and not to children with more recent diagnoses. Cerebral or cervical arteriopathies are the strongest predictor of recurrent stroke in children8; however, we had no access to imaging, including vascular imaging, so could not assess whether a cerebral arteriopathy was on the causal pathway between radiation and recurrent stroke. Finally, although we collected data on antithrombotic use, because we could not reliably distinguish ischemic from hemorrhagic stroke, and because of concerns for confounding by indication, we could not perform a meaningful analysis of efficacy of such therapy in reducing risk of recurrent ischemic stroke.
Dramatic improvements in survival rates of childhood cancers make more apparent the later effects of life-saving cancer therapies. Stroke is a particularly devastating consequence that can not only lead to physical disability, but can worsen the cognitive impairment that many survivors develop as a direct effect of cranial radiation therapy, or the cancer itself. A better understanding of risk factors for stroke, and the underlying mechanisms, creates opportunities for primary and secondary stroke prevention. A role for accelerated atherosclerosis suggests particularly low-hanging fruit for risk modification in this population: the early identification and treatment of modifiable atherosclerotic risk factors like hypertension. Screening guidelines might need to be adjusted to assess for these risk factors in a more uniform way in this at-risk population.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants and their families for their participation in the CCSS and additional stroke survey.
GLOSSARY
- CCSS
Childhood Cancer Survivor Study
- CI
confidence interval
- IQR
interquartile range
- IRB
institutional review board
- NDI
National Death Index
- NF1
neurofibromatosis type 1
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
H.J. Fullerton: involved in design and conceptualization of the study, analysis and interpretation of the data, and drafting the manuscript. K. Stratton: involved in design and conceptualization of the study, analysis and interpretation of the data, and drafting the manuscript. S. Mueller: involved in design and conceptualization of the study, analysis and interpretation of the data, and drafting the manuscript. W. Leisenring: involved in design and conceptualization of the study, analysis and interpretation of the data, and drafting the manuscript. G.T. Armstrong: involved in design and conceptualization of the study and drafting the manuscript. R.E. Weathers: involved in design and conceptualization of the study and drafting the manuscript. M. Stovall: involved in design and conceptualization of the study, analysis and interpretation of the data, and drafting the manuscript. C.A. Sklar: involved in design and conceptualization of the study and drafting the manuscript. R.E. Goldsby: involved in design and conceptualization of the study and drafting the manuscript. L.L. Robison: involved in design and conceptualization of the study, analysis and interpretation of the data, and drafting the manuscript. K.R. Krull: involved in design and conceptualization of the study, analysis and interpretation of the data, and drafting the manuscript.
STUDY FUNDING
Supported by the National Cancer Institute (U24 CA 55727 to L.L.R.), Cancer Center Support (CORE) (grant CA21765), and the American Lebanese-Syrian Associated Charities (ALSAC). Dr. Mueller and Dr. Fullerton were supported by a private donation from the LaRoche family, and Dr. Mueller by the National Center for Advancing Translational Sciences, NIH, through UCSF-CTSI grant number KL2TR000143, and the Frank A. Campini Foundation.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2006;24:5277–5282. [DOI] [PubMed] [Google Scholar]
- 2.Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys 2013;86:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation. Int J Radiat Oncol Biol Phys 2013;86:643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddy N, Mousannif A, Tukenova M, et al. Relationship between the brain radiation dose for the treatment of childhood cancer and the risk of long-term cerebrovascular mortality. Brain 2011;134:1362–1372. [DOI] [PubMed] [Google Scholar]
- 5.Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke 2012;43:3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouladi M, Langston J, Mulhern R, et al. Silent lacunar lesions detected by magnetic resonance imaging of children with brain tumors: a late sequela of therapy. J Clin Oncol 2000;18:824–831. [DOI] [PubMed] [Google Scholar]
- 7.Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke 2011;42:2410–2418. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics 2007;119:495–501. [DOI] [PubMed] [Google Scholar]
- 9.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009;27:2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol 2002;38:229–239. [DOI] [PubMed] [Google Scholar]
- 12.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons; 1980. [Google Scholar]
- 14.Putaala J, Haapaniemi E, Kurkinen M, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts, leukoaraiosis, and long-term prognosis in young ischemic stroke patients. Neurology 2011;76:1742–1749. [DOI] [PubMed] [Google Scholar]
- 15.Kappelle LJ, Adams HP, Jr, Heffner ML, Torner JC, Gomez F, Biller J. Prognosis of young adults with ischemic stroke: a long-term follow-up study assessing recurrent vascular events and functional outcome in the Iowa Registry of Stroke in Young Adults. Stroke 1994;25:1360–1365. [DOI] [PubMed] [Google Scholar]
- 16.Omura M, Aida N, Sekido K, Kakehi M, Matsubara S. Large intracranial vessel occlusive vasculopathy after radiation therapy in children: clinical features and usefulness of magnetic resonance imaging. Int J Radiat Oncol Biol Phys 1997;38:241–249. [DOI] [PubMed] [Google Scholar]
- 17.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation 2006;114:2170–2177. [DOI] [PubMed] [Google Scholar]
- 18.Waddy SP, Cotsonis G, Lynn MJ, et al. Racial differences in vascular risk factors and outcomes of patients with intracranial atherosclerotic arterial stenosis. Stroke 2009;40:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 20.Muzaffar K, Collins SL, Labropoulos N, Baker WH. A prospective study of the effects of irradiation on the carotid artery. Laryngoscope 2000;110:1811–1814. [DOI] [PubMed] [Google Scholar]
- 21.De Bruin ML, Dorresteijn LD, van't Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst 2009;101:928–937. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol 2014;32:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.