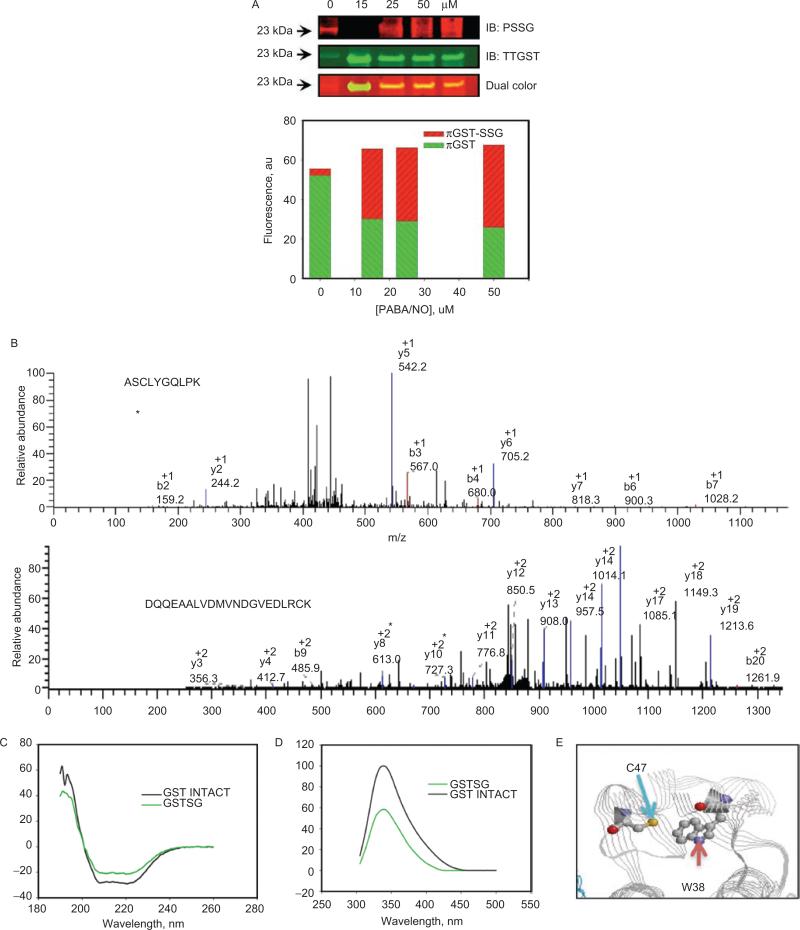
Figure 3.
Autoregulation of GSTP occurs through the S-glutathionylation of Cys47 and Cys101. HEK293 cells were treated with 0–50 uM of PABA/NO for 1 hour (A). The proteins were separated by nonreducing sodium dodecyl sulfate polyacrylamide electrophoresis, and S-glutathionylation was evaluated by immunoblot with PSSG monoclonal primary antibody and GSTP polyclonal primary antibody and detected simultaneously with both red (antimouse) and green (antirabbit) fluorescent secondary antibodies. The dual-colored image was quantified, and the bars represent a relative input of red and green fluorescence in each band. Matrix-assisted laser desorption/ionization mass spectrometric analysis of purified, expressed GSTP treated with 50 μM of PABA/NO and 0.5 mM of GSH showed that peptides containing Cys47 and Cys101 are S-glutathionylated (B). S-glutathionylation of Cys47 and Cys101 on GSTP alters structure. Spectroscopic analysis of native (black) and PABA/NO+GSH-treated (green) GSTP in vitro was performed using CD (C) and tryptophanyl fluorescence (D) of purified protein. According to the published crystal structure (Ji et al., 1997), the relative positions of GSTPs C47 and W38 are depicted (E) using RasMol 2.7.4.2 (http://rasmol.org last accessed February 18 2011). From Townsend et al., (2009a).