Fig. 1.
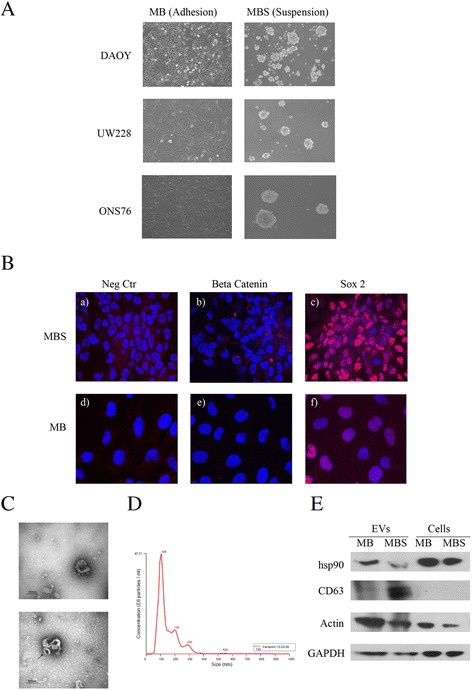
Purification of microvescicles from medulloblastoma cell lines and medulloblastoma spheres. a MB cell lines DAOY, UW228 and ONS-76 were cultured both in adhesion (MB) and as spheres (MBS). MBS were plated in ultra-low attachment flasks in EUROMED CSC serum-free medium (Euroclone code: ECM0894D). 4X magnification. b Representative image of immunofluorescence staining at 63X magnification of MB and MBS: (a, d) negative control, (b,e) β catenin (red), (c,f) Sox2 (red). c Electron microscopy representative images of exosomes from Medulloblastoma (MB) and Medulloblastoma spheres (MBS) from DAOY cells purified by ultracentrifugation. Note the cup-shaped morphology of vesicles (a). An aggregate of three exosomes (arrow) is clearly evident compared with smaller non cup-shaped vesicles in the background (b). d Nanoparticle tracking analysis (NTA) of MB and MBS EVs using the NanoSight instrument. Plot shows EV particle size distribution profiles and concentration measurements. The NanoSight instrument is based on a conventional optical microscope and uses a laser light source to illuminate nano-scale particles. e Western blot analysis of EV extracts from MB (adhesion), MBS (spheres) and of cells originating from EVs. Antibodies against HSP90, Actin and CD63 were used. CD63 is a specific marker of microvesicles (MVs). Actin was used as a loading control
