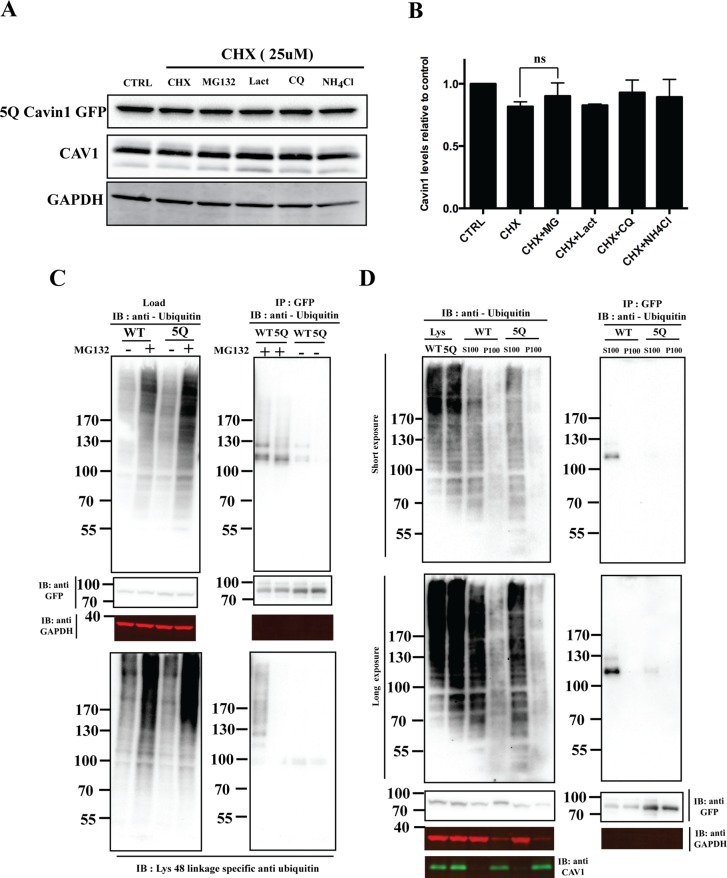
FIGURE 3:
5Q mutation significantly reduces cavin1 ubiquitylation and is less sensitive to proteasomal inhibitors. (A) PC3 cells were transiently transfected with 5Q cavin1 mutant and were treated with 25 μM CHX in the presence or absence of MG132 (10 μM), Lact (10 μM), CQ (10 μM), or NH4Cl (10 mM) for 6 h and were subsequently immunoblotted for GFP, CAV1, and GAPDH. (B) Quantification of cavin1-GFP levels from immunoblot analysis of three independent experiments. Each bar represents mean and error bars represent SD. ns, no significant difference. (C) A431 cells were transiently transfected with WT cavin1-GFP or 5Q cavin1-GFP and treated with either MG132 or dimethyl sulfoxide. Cell lysates were immunoprecipitated for GFP with GFP nanobeads. Then lysates and immunoprecipitated samples were immunoblotted for GFP, GAPDH, and antiubiquitin antibody to detect total ubiquitylated proteins and Lys-48 linkage–specific antiubiquitin antibody to detect ubiquitylated species of cavin1 specifically attached by the Lys-48 residue of ubiquitin that marks target protein for proteasome degradation. (D) A431 cells were transiently transfected with WT cavin1-GFP or 5Q cavin1-GFP and treated with MG132 for 2 h. Subsequently cells were lysed in buffer C and subjected to ultracentrifugation to separate the membrane fraction as a pellet (P100) and supernatant (S100). Further, GFP immunoprecipitation was performed by dissolving the membrane fraction in buffer B and immunoblotting for GFP, GAPDH, CAV1, and with antiubiquitin to detect ubiquitylated species of cavin1. Representative uncropped immunoblots are shown in Supplemental Figure S2.