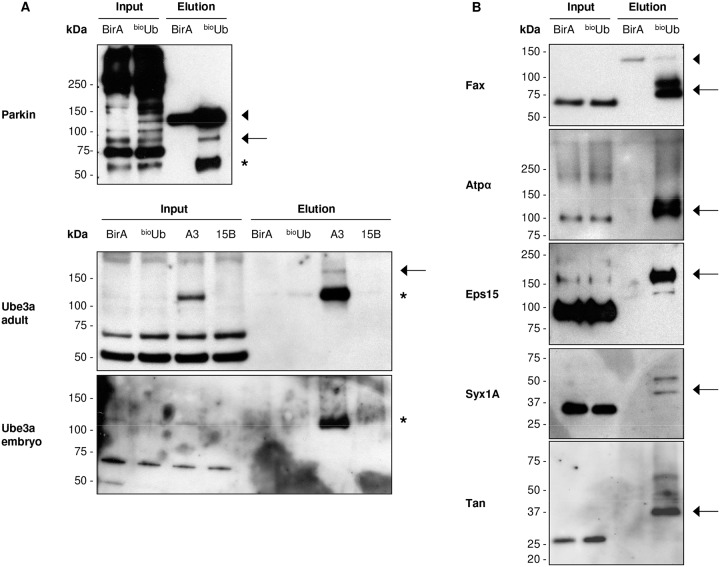
Fig 3. Western blot validation of identified ubiquitin conjugates and ubiqutin carriers.
(A) Western blot performed with antibodies against Parkin (upper panel) or Ube3a E3 ligases (bottom panels). The ability of the HECT-type, as well as RING between RING-type E3 ligases to form thioester linkages with the ubiquitin before they transfer it to the substrates allow us to trap them while they are carrying ubiquitin. Since the reducing agents used to perform the elution from the beads breaks this type of linkage, there is no increase in their molecular weight relative to the inputs (*). A fraction of both E3s appears also conjugated with ubiquitin despite the DTT (arrows). This is probably due to auto-ubiquitination at some lysine residue. Parkin antibody non-specifically recognized several proteins in the inputs. The appropriate Parkin band is the one at 55 kDa. (B) Western blots with specific antibodies to some of the proteins identified in the adult pulldown revealed the expected increase of their molecular weight in the bioUb sample relative to the inputs. Covalent attachment of ubiquitin should increase the protein’s molecular weight by about 10 kDa for each ubiquitin attached. Therefore, the increase shown in the Western blots (arrows) reflects their ubiquitinated status. Endogenous biotinylated CG1516 protein, non-specifically identified by some antibodies, is marked with an arrowhead. All Western blots were performed with adult samples, except Ube3a that was also test in embryo. BirA: GMR GAL4 /CyO;UAS BirA/TM6; bioUb: GMR GAL4,UAS ( bio Ub) 6 -BirA/CyO; A3 (Ube3a overexpression): GMR GAL4,UAS ( bio Ub) 6 -BirA/CyO;UAS Dube3A/TM6B; 15B (Ube3a deletion mutant): GMR GAL4,UAS ( bio Ub) 6 -BirA/CyO, Dube3A 15B /TM6B.