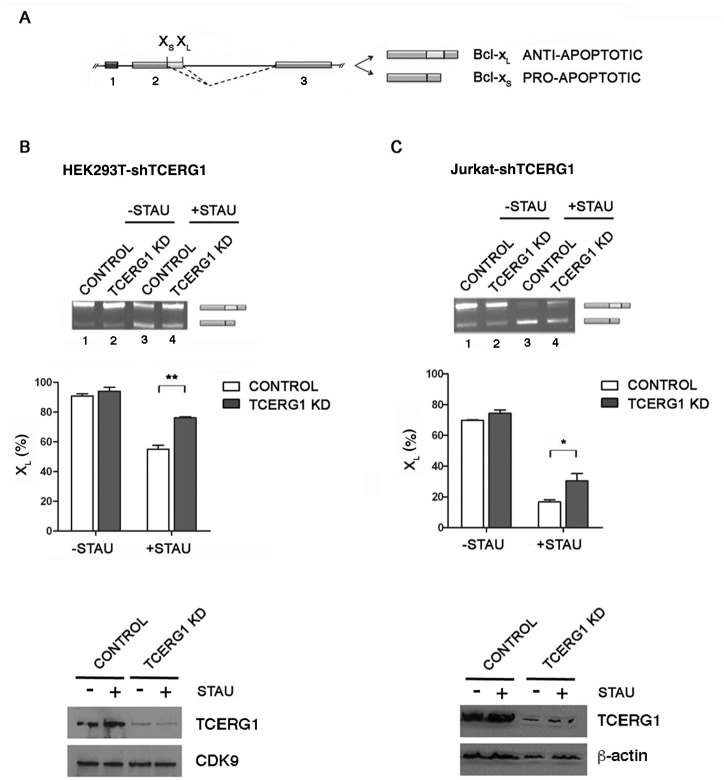
Fig 1. Regulation of Bcl-x alternative splicing by TCERG1 upon staurosporine-induced apoptosis.
(A) Schematic representation of the structure of the Bcl-x gene, with exons (boxes) and introns (lines). Two splice variants derived from the Bcl-x gene, anti-apoptotic Bcl-xL and pro-apoptotic Bcl-xS, are generated via alternative 5’ splice site (XS and XL) selection within exon 2. The dotted lines indicate the alternative splicing events. (B and C) Knockdown of TCERG1 (KD) increased the level of the anti-apoptotic Bcl-xL isoform after staurosporine treatment. Control (lanes 1 and 3) and TCERG1 knockdown T-Rex-HEK293T cells (lanes 2 and 4) (B) and Jurkat cells (C) were treated with 1 μM staurosporine for 12 h and 0.5 μM staurosporine for 1 h, respectively. After total RNA extraction, the RNA splicing variants were amplified by RT-PCR, and the products were separated on 2% agarose gels. The graphs show the densitometric results as the percentage of the Bcl-xL isoform in three independent experiments (means ± SEM). *, p < 0.05, **, p < 0.01. A fraction of the cell lysates was analyzed by immunoblotting for TCERG1, CDK9, and ß-actin.