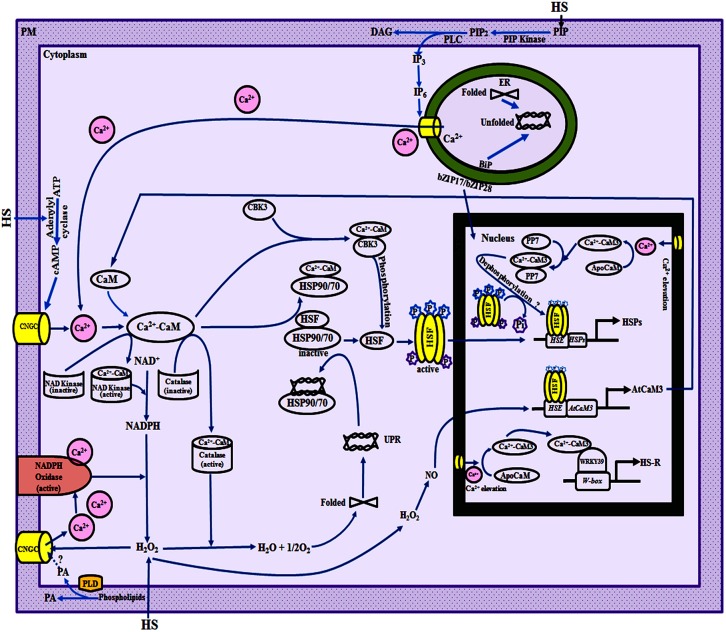
Figure 2.
A model illustrating the role of Ca2+/calmodulin (CaM) in regulation of heat shock response and H2O2 homeostasis in plants. Changes in plasma membrane (PM) fluidity due to heat stress result in activation of phospholipase D (PLD) and phosphatidylinositol-4-phosphate 5-kinase (PIP kinase), and consequently the accumulation of various lipid signaling molecules such as phosphatidic acid (PA) and phosphatidylinositol-4, 5-bisphosphate (PIP2; Mishkind et al., 2009). Thermal stress also activates phospholipase C which converts PIP2 into diacyl glycerol (DAG) and D-myo-inositol-1,4,5-trisphosphate (IP3). IP3 may be phosphorylated and converted into IP6 that interacts with endoplasmic reticulum (ER)-localized Ca2+-channels thus resulting in release of Ca2+ from intracellular stores (Mishkind et al., 2009; Mittler et al., 2012). Rapid influx of extracellular Ca2+ in the cell can also occur due to temperature-induced activation of the PM-localized cyclic nucleotide gated channels (CNGCs) which are non-selective inward cation channels. The CNGC may be activated by heat stress-induced rapid burst in H2O2 levels (Pei et al., 2000) by cyclic adenosine monophosphate (cAMP) that is produced by heat stress-activated adenylyl cyclase (Köhler et al., 1999) and/or by PA (Mittler et al., 2012). The thermal stress-induced increase in [Ca2+]cyt leads to conversion of ApoCaM to Ca2+-CaM. The expression of HSP genes is repressed in the absence of heat stress which is proposed to be due to interaction of heat shock factors (HSFs) with HSP90 (Zou et al., 1998) and/or HSP70 (Sun et al., 2000). The Ca2+-CaM and the denatured proteins, produced as a result of unfolded protein response (UPR) due to reactive oxygen species (ROS)-mediated oxidation, bind to HSP70/HSP90, thereby releasing the HSFs (Yamada and Nishimura, 2008; Virdi et al., 2009, 2011). H2O2 acts upstream of NO which regulates the expression of AtCaM3 through modulation of the binding of HSF to the heat shock elements (HSEs; Wang et al., 2014a). The temperature-induced increase in NO leads to enhanced levels of AtCaM3, that then binds to Ca2+ and activates the protein kinase AtCBK3 (Arabidopsis thaliana Ca2+-CaM-binding Kinase 3), resulting in phosphorylation and trimerization of HSFs which are then translocated to the nucleus (Queitsch et al., 2000). The interaction of activated HSFs with HSEs leads to synthesis of HSPs. Dephosphorylation of the HSFs at selected amino acid residues by a nuclear-localized Ca2+-CaM-binding phosphatase (PP7) may lead to continuous activation of heat shock-regulon (HSR) but the precise mechanism is still not understood. The heat shock response through Ca2+-CaM-mediated regulation of WRKY transcription factors appears to be independent of HSF-mediated pathway. The WRKY39, after binding to Ca2+-CaM, interacts with the W-box elements present in the upstream promotor regions of different genes involved in thermotolerance. The ER also plays a critical role in thermal adaptation through UPR-induced release of ER membrane-tethered transcription factors such as bZIP17/bZIP28/bZIP60, which after release are translocated to the nucleus and activate the transcription of genes encoding ER-chaperones and brassinosteroid-signaling pathway related genes (Che et al., 2010; Deng et al., 2011). The intracellular levels of H2O2 under stress also appear to be maintained through Ca2+-CaM (Wang et al., 2014a). The H2O2-induced activation of CNGCs (Pei et al., 2000) results in an increase in the [Ca2+]cyt (Price et al., 1994) that further activates NADPH oxidase that converts NADPH to H2O2 (Keller et al., 1998). The conversion of NAD+ to NADPH is catalyzed by NAD kinase that is also modulated through Ca2+-CaM (Harding et al., 1997). These observations, therefore, suggest the presence of an intricate feedback regulation through Ca2+/CaM pathway which allows the plants to maintain H2O2 homeostasis. BiP, binding immunoglobulin protein; ER, endoplasmic reticulum; HS, heat stress; NE, nuclear envelop; PM, plasma membrane.