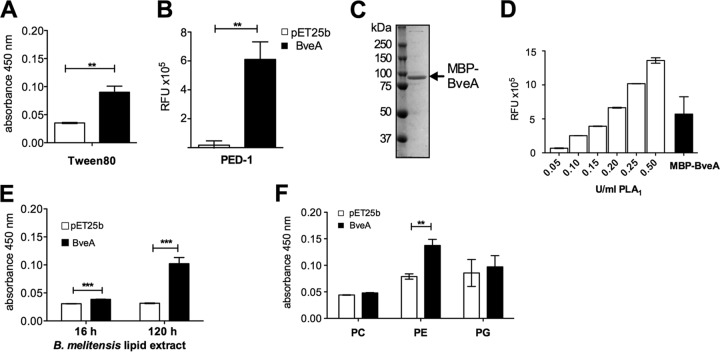
FIG 2.
BveA is a phospholipase A1 with specificity for PE lipase activity against Tween 80 (A) and the phospholipase A1 substrate PED-1 (B) using periplasmic fractions containing BveA and the empty vector control. Purification (C) and lipase activity (D) of the MBP-BveA fusion protein (97-kDa) compared to those of increasing units of gelatinase, a positive control for phospholipase A1 activity (PLA1) (Life Technologies). Lipase activity of BveA against lipid extractions of the B. melitensis cell envelope (E) and against phospholipid standards (Sigma-Aldrich) PC, PE, and PG (F). Significant differences between BveA-containing samples and the empty vector control: **, P < 0.01; ***, P < 0.001. RFU, relative fluorescence units.