Figure 6.
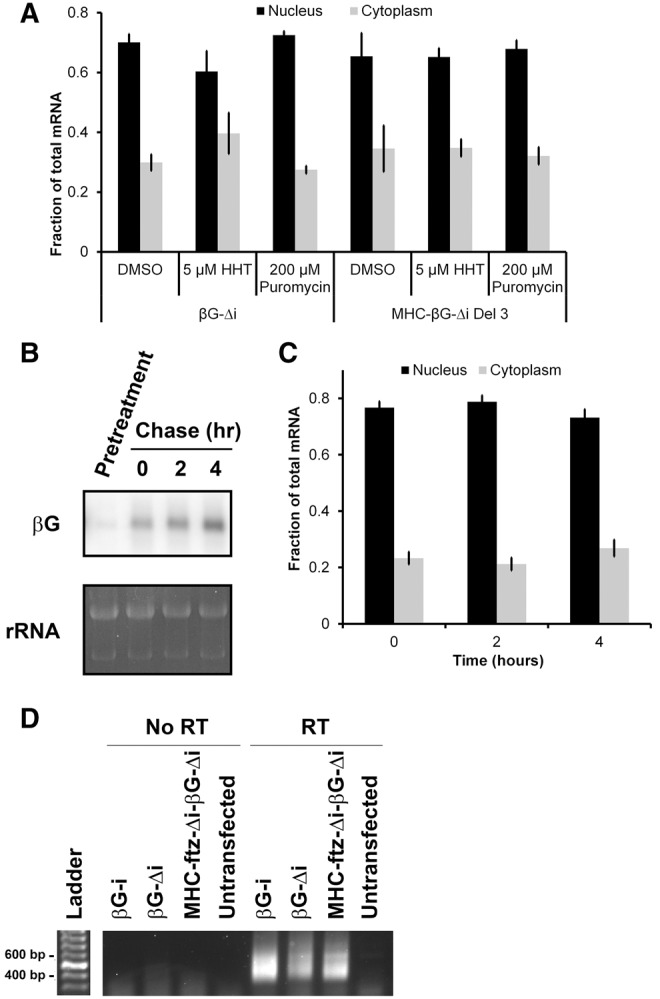
The majority of the βG-Δi mRNA that is present at steady state is stable and retained in the nucleus. (A) To test whether βG-Δi is a substrate for NMD, U2OS cells were transfected for 6 h, then treated with either DMSO, puromycin, or HHT at levels that completely inhibit translation (see Cui et al. 2012) for a further 8 h. Cells were then fixed, permeabilized, stained for mRNA using a FISH probe directed against βG for βG-Δi mRNA, or MHC SSCR for MHC-βG-Δi Del 3, and the levels of nuclear and cytoplasmic RNA were quantified (B,C). U2OS cells were transfected for 14 h, then treated with α-amanitin for the indicated times. Then, either RNA was collected and analyzed by Northern blot using βG-specific probes (B) or cells were fixed, permeabilized, stained for mRNA using a FISH probe directed against βG and the levels of nuclear and cytoplasmic RNA were quantified (C). To ensure that the α-amanitin inhibited transcription, cells were first treated with drug, then transfected (“Pretreatment”). Total RNA (mostly comprising of rRNA) was stained with ethidium bromide as a loading control. Each bar in A and C represents the average and standard error of three independent experiments, each of which consists of at least 30 cells. (D) Cells were transfected with the indicated constructs and RNA was isolated after 14 h. To ensure that the specificity of the detected signal, RNA was also isolated from untransfected cells. The length of their poly(A) tails was assessed using ePAT as described previously (Jänicke et al. 2012). Reverse transcription reactions were performed with either the reverse transcriptase added, “RT”, or not added to the reactions, “No RT.”
