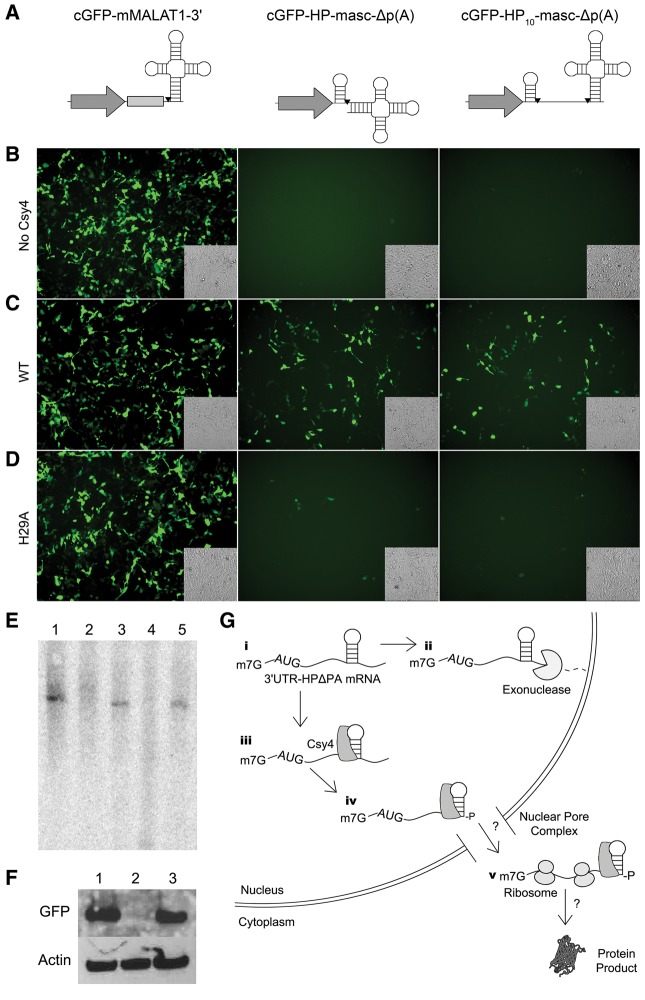
FIGURE 4.
Selective processing by Csy4 is essential for rescue of 3′ UTR-HP-Δp(A) constructs. (A) Schematics of the unmodified cGFP-mMALAT1-3′ reporter (left), cGFP-HP-masc-Δp(A) (middle), and cGFP-HP10-masc-Δp(A) (right) reporters. Cleavage sites are indicated by black inverted triangles. Fluorescent images of HEK293 cells expressing either cGFP-mMALAT3′ (left), cGFP-HP-masc-Δp(A) (middle), or cGFP-HP10-masc-Δp(A) (right) in the absence of Csy4 (B), with Csy4 wild type (C), or Csy4-H29A (D). Corresponding transmitted light images are shown as insets in each fluorescent image. (E) Northern blot of cGFP-based reporters in the presence or absence of Csy4. cGFP-mMALAT1-3′ (lane 1), cGFP-HP-mascΔp(A) (lane 2), cGFP-HP-mascΔp(A) with Csy4 (lane 3), cGFP-HP10-masc-Δp(A) (lane 4), and cGFP-HP10-masc-Δp(A) with Csy4 (lane 5). (F) A Western blot probing for cGFP using cell lysates of HEK293s expressing either cGFP-mMALAT1-3′ (lane 1), cGFP-HP-mascΔp(A) (lane 2), or cGFP-HP-mascΔp(A) with Csy4 (lane 3). A Western blot for Actin is provided as a loading control. (G) Potential events outlining Csy4-mediated processing of 3′ UTR-HP-Δp(A) mRNA. First, within the nucleus, poly(A)-deficient constructs are likely degraded by cellular exonucleases in the absence of Csy4 [steps (i) and (ii)]. However, Csy4 binding (iii) followed by cleavage of the substrate (iv) appears to stabilize the mRNA and enable cytosolic transport as well as translation (v) by unknown mechanisms.