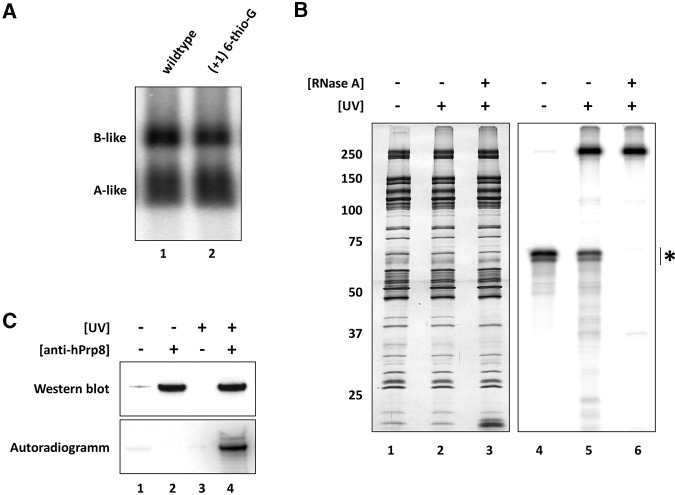
FIGURE 6.
Exon–intron junction nucleotides of the 5′ss oligo interact with the hPrp8 protein in 45S B-like complexes. (A) The (+1) 6-thio-G modification does not hinder B-like complex formation as assayed by native gel electrophoresis performed as previously described. (B) 45S B-like complexes assembled in the presence of a 5′-32P-labeled (+1) 6-thio-G modified 5′ss oligo were subjected to glycerol-gradient centrifugation, and affinity-purified complexes were irradiated at 365 nm (lanes 2,3,5,6) to induce protein–RNA crosslinks and subsequently treated with RNase A (lanes 3,6). Proteins were separated on a 10%–13% polyacrylamide gel and visualized by silver staining (lanes 1–3) or by a phosphoimager (lanes 4–6). (*) Position of MINX exon–RNA. (C) Immunoprecipitation confirms that the major crosslinked protein is hPrp8. 45S B-like complexes ± UV irradiation were denatured and immunoprecipitation was performed with PAS-bound antibodies against hPrp8, as indicated. Immunoprecipitated proteins were analyzed by Western blotting with hPrp8-specific antibodies (upper panel) and proteins crosslinked to the 32P-labeled 5′ss oligo were detected by autoradiography (lower panel).