Figure 1.
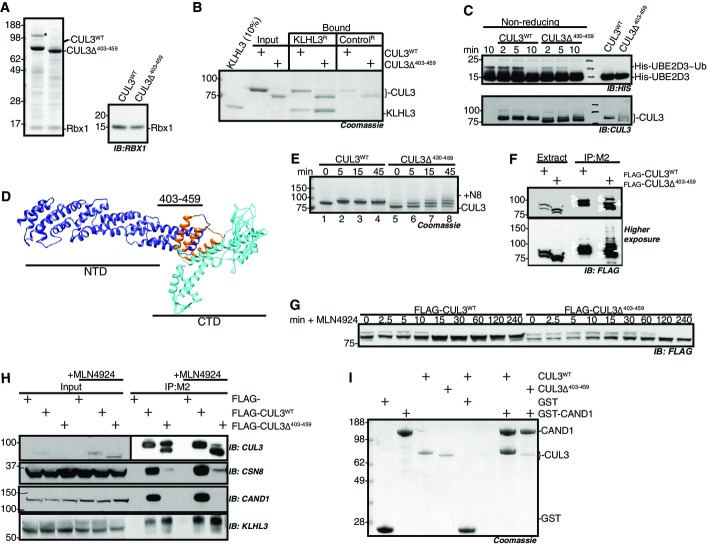
- Purified CUL3WT and CUL3Δ403–459 analysed by SDS–PAGE and Coomassie blue staining, following expression in SF21 cells, and DAC-tag-affinity purification and tag cleavage (see Materials and Methods). Bands migrating between 62- and 98-kDa protein markers are CUL3WT and CUL3Δ403–459, respectively, while the band below 17 kDa is Rbx1. The immunoblot for RBX1 is shown on the right. The band in CUL3WT denoted with * is co-purified insect cell CAND1, which was later removed by SEC. All protein identities were confirmed by mass spectrometry.
- KLHL3 binding to CUL3WT and CUL3Δ403–459 is comparable as shown by pull-down assays with a KLHL3 resin generated from cross-linking anti-KLHL3 antibody to Protein G sepharose, and then binding saturating amounts of KLHL3 to the anti-KLHL3 resin (denoted KLHL3R), the control resin (ControlR) is Protein G sepharose (see Materials and Methods for further detail). CUL3WT or CUL3Δ403–459 was incubated with either KLHL3R or ControlR at 4°C for 1 h and then washed thrice with 1× PBS, resin volumes were minimised, and SDS–Laemmli buffer was used to elute protein from resin prior to analysis by SDS–PAGE and staining with colloidal blue.
- UBE2D3˜ubiquitin-release assays analysed by non-reducing SDS–PAGE and immunodetection. His-tagged UBE2D3 was charged with ubiquitin by incubation with ATP and UAE, and charging was stopped by ATP depletion with apyrase. No CUL3 (negative control, lane 1), CUL3WT or CUL3Δ403–459 was then added to the charged E2 and the activity of the RING domain, RBX1, monitored by the hydrolysis of ubiquitin from UBE2D3, visualised by immunodetection with anti-His antibody (upper panel, “˜” denotes thioester bond). The lower panel is an immunoblot for CUL3, showing equivalent amount of both CUL3WT and CUL3Δ403–459 was added to the reaction mix; over time, the appearance of higher molecular weight bands represents the modification of CUL3 by covalent attachment of ubiquitin. The right two lanes are control lanes incubated for 10 min with E2˜Ubi but then treated with reducing agent prior to SDS–PAGE.
- Structural model of CUL3WT based on the structure of CUL1 (PDB:1LDK), highlighting the residues deleted in the PHA2E mutant which are located close to the N-terminal domain (NTD) and C-terminal domain (CTD) border, using Phyre2 and Chimera (see Materials and Methods). The NTD is coloured purple, the CTD cyan, and residues 403–459 orange.
- Time course of in vitro neddylation reactions. Purified NAE, UBE2M and Nedd8 were mixed with either CUL3WT or CUL3Δ403–459 and incubated at 30°C for the time indicated. Due to the covalent attachment of Nedd8 (8.6 kDa), modified proteins have decreased mobility. The band shift observed for CUL3WT (lane 1 versus lane 2) is representative of a single Nedd8 modification, while the multiple bands in the CUL3Δ403–459 reaction reflect the attachment of up to three Nedd8 molecules (lane 5 versus lane 8).
- HEK-293 cells over-expressing either FLAG-CUL3WT or FLAG-CUL3Δ403–459 were immunoprecipitated with M2 Flag-binding sepharose, in the presence of a deneddylase inhibitor (OPT). Extract, cells were lysed in the presence of OPT and clarified by centrifugation. IP:M2, clarified supernatant samples were incubated for 1 h at 4°C with M2 (anti-Flag) sepharose, then washed with PBS and eluted with SDS–Laemmli buffer, prior to SDS–PAGE and immunodetection with anti-FLAG antibody. Bands are labelled as follows: 1. CUL3WT, 2. CUL3WT-N8, 3. CUL3Δ403–459, 4. CUL3Δ403–459-N8, 5. CUL3Δ403–459-2(N8).
- Neddylation in HEK-293 cells over-expressing FLAG-CUL3WT or FLAG-CUL3Δ403–459 was blocked by treatment with a Nedd8-E1-enzyme inhibitor, 3 μM MLN4924 (Millennium Pharmaceuticals), in cells over-expressing FLAG-CUL3WT and FLAG-CUL3Δ403–459 respectively. Cell extracts were sampled over time in the presence of OPT to prevent de-neddylation and subjected to SDS–PAGE and immunodetection of the FLAG tag.
- FLAG-CUL3WT or FLAG-CUL3Δ403–459 was immunoprecipitated with M2-sepharose as described in (F). +MLN4924 indicates cell culture media supplementation with 3 μM MLN4924 for 5 h prior to cell lysis to achieve complete deneddylation of CUL3. Following SDS–PAGE, immunoblotting with COP9 Signalosome (CSN) or CAND1 antibodies determined the interaction between these Cullin regulators and FLAG-CUL3WT and FLAG-CUL3Δ403–459, respectively.
- GST-CAND1 pull-down assays with CUL3 proteins. Purified GST or GST-CAND1 immobilised on glutathione–sepharose was mixed with either purified CUL3WT or CUL3Δ403–459 and incubated at 4°C for 1 h. The sepharose was then washed thrice and eluted from the resin by incubation with SDS–Laemmli buffer prior to SDS–PAGE and Coomassie staining. CUL3WT but not CUL3Δ403–459 interacts with CAND1.
Source data are available online for this figure.