Figure 3.
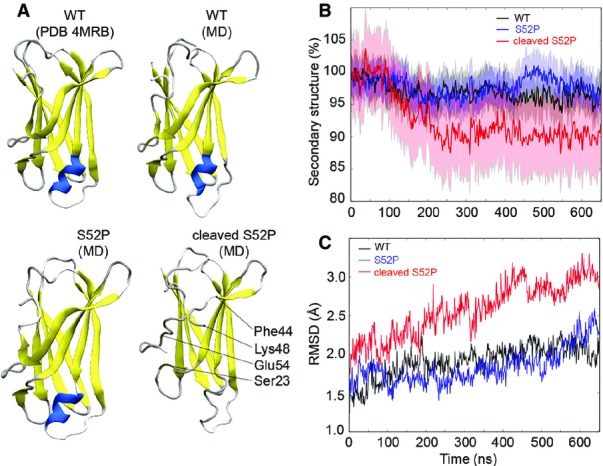
Cleavage at residue Lys48 induces an allosteric conformational change
- Structures of wild-type and S52P subunits after 500-ns MD simulation are similar to the original wild-type crystal structure (Mangione et al, 2014). Conversely, the cleavage of S52P TTR induces an unfolding in the 43–48 strand, followed by loss of secondary structure in the Ser23, Glu54 regions and the EF-helix (in blue), respectively.
- Relative percentage of secondary structure retained during the simulations of wild-type (black) and of the S52P variant TTR with (red) and without (blue) cleavage. The solid lines show average values measured in four independent subunits. The full colour areas represent standard deviations.
- Root mean square deviations during the simulations of wild-type (black) and S52P variant TTR before (blue) and after (red) cleavage.
