Abstract
Stress is a major factor for a risk of cerebrovascular catastrophes. Studying of mechanisms underlying stress-related brain-injures in neonates is crucial for development of strategy to prevent of neonatal stroke. Here, using a model of sound-stress-induced intracranial hemorrhages in newborn rats and optical methods, we found that cerebral veins are more sensitive to the deleterious effect of stress than arteries and microvessels. The development of venous insufficiency with decreased blood outflow from the brain accompanied by hypoxia, reduction of complexity of venous blood flow and high production of beta-arrestin-1 are possible mechanisms responsible for a risk of neonatal hemorrhagic stroke.
OCIS codes: (170.0170) Medical optics and biotechnology, (170.3880) Medical and biological imaging, (170.1470) Blood or tissue constituent monitoring, (170.2655) Functional monitoring and imaging
1. Introduction
Neonatal stroke is among the top ten causes of death (mortality rate is approximately 25%) in newborns [1]. In recent years, it has been believed that the advanced age is the key risk factor for the stroke. The clinical data suggest that 95% of stroke occurs in people older 45, more than 50% of strokes occur in people over the age of 65 [2]. However, during the last decade due to improvement of neuroimaging, it has become obvious that the stroke can often occur even in newborns and the incidence of neonatal stroke is similar to adult stroke [3–5]. The incidence of neonatal stroke in term newborns is commonly referred to as 1.17/1000 live births [4, 6]. The incidence of stroke in preterm infants (i.e., who are born earlier normal time with low birth weight less 1500 g) has declined up 45% [7]. But the true incidence is possibly higher because many cases of neonatal stroke are presented usually without any outward clinical symptoms or with non-specific neurological signs [4,8,9]. The long-term clinical observations in small patients who were diagnosed suggest that in further life, major cognitive deficit develops in more than 50% of neonates after the stroke and approximately 75% of such babies need special education in school [10–13]. Thus, neonatal stroke is a major problem of future generation’s health due to the high rate of death and cognitive disability of newborns after the stroke. Therefore, the study of hidden mechanisms underlying the silent pathological processes preceding and accompanying the stroke in neonates is absolutely essential
In contrast to adults, about half of strokes in newborns are hemorrhagic [14]. Reasons and mechanisms underlying neonatal hemorrhagic stroke (NHS) are difficult to determine. Potential risk factors for NHS are still poorly studied. Some works report an association between assisted deliveries and the stroke in neonates, but those results are not consistent [15,16]. Such risk factors as hypertension, smoking, diabetes, and atherosclerosis are not suitable for neonates.
Major risk factor for NHS is stress, which babies have during the crucial physiological changes during delivery and intensive adaptation to the new conditions of life. The first 3 days of life are most critical, up to 50% of newborns die within this stressful period [17,18]. In experimental work, it was clearly shown that early neonatal stress such as the mother-infant separation significantly increases a risk for the stroke [19]. However, the role of stress in neonatal stroke is not well studied due to methodological difficulties. The existing animal models of hemorrhagic stroke have significant limitations [20,21]. The traditional methods for inducing of hemorrhagic stroke are the direct injection of blood, its components, or bacterial collagenase into the brain parenchyma that only mechanically mimics the rupture of cerebral vessels but does not allow studying the stress processes preceding the stroke.
For revealing of markers of the pre-stroke, in this study on three-day-old pups we used a non-invasive model of stress-related hemorrhagic stroke induced by severe sound stress [22,23]. Despite the fact that the sound is not original factor for NHS, this model mimics early pathological mechanisms preceding NHS that are closely to clinical evens. Indeed, the sound-brain damages develop with the latent changes in cortical and subcortical brain tissues which is close to localization as well as origin time of NHS in term neonates [4,5,24,25].
For the better understanding of the role of stress in the development of stroke in babies during the first days after birth, the detailed studies in this field are essential. It can help to prevent the harmful effect of stress on the immature neonatal brain during critical period of life.
Here, we focused on different levels of the cascade of stress responses: morphological (histological analysis of brain-injures), systemic (monitoring of cerebral blood flow (CBF)), inter-systemic (a complexity measure of CBF), metabolic (assessment of oxygen saturation of the brain tissues) and molecular (evaluation of beta-arrestin-1level). Our choice of experimental tasks is caused by main actual questions related to the stress-induced cerebrovascular catastrophes. Many studies showed that abnormalities of the CBF, including fluctuations in the CBF, provide a considerable contribution to the neonatal stroke [26–28]. Perinatal cerebral hypoxia plays an important role in the pathology of stroke in newborns and in the modulation of molecular mechanisms of vascular stress-reactivity such as beta-arrestin-1 [29,30]. But, the exact role of these processes in NHS, especially, in aspect of stress accompanying the birth is not established yet and needs to be thoroughly studied.
2. Methods
Experiments were carried out in newborn mongrel rats 3 days old (n = 37). The animals were divided into three groups: 1) intact, unstressed newborn rats (the control group, n = 10); 2) stressed rats 4 h after stress-off (the pre-stroke group, rats with initial pathological changes in the brain tissues and cerebral circulation, n = 15); stressed rats 24 h after stress-off (the post-stroke group, rats with intracranial hemorrhages, n = 12). All procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” [31]. The experimental protocol was approved by the Committee for the Care and Use of Laboratory Animals at Saratov State University (Protocol H-147, 17.04.2001).
To induce hemorrhagic stroke, the following protocol of sound stress’s impact was used (120 dB, 17 Hz): 10 sec – sound, then 60 sec – pause and this cycle repeated during 2 h [22,23]. To analyze the brain-injures induced by stress and to confirm the development of stroke, all newborn rats were decapitated for a histological study of brain tissue. The samples were fixed in 10% buffered neutral formalin. The formalin fixed specimens were embedded in paraffin, sectioned (4 µm) and stained with haematoxylin and eosin.
To assess the stress-related changes in cerebral circulation, we studied the changes in relative cerebral blood flow (rCBF or perfusion) using a home-made system for laser speckle contrast imaging (LSCI). The raw speckle images were recorded under the following conditions: light source – HeNe laser with the wavelength 632.8 nm; image sensor – CMOS camera Basler acA2500-14gm; imaging lens – Computer M16140-MP2 16 mm at F-number equal to 6, that corresponds to speckle/pixel size ratio of around 2; exposure time – 20 ms. The speckle images were recorded for 3 min at an average frame rate of 40 frames per second. Spatial speckle contrast was calculated as , where σ is the standard deviation of intensity fluctuations and is the mean intensity within a sliding window. Fifty consecutive frames were averaged into one speckle contrast image.
To analyze the velocity of blood flow in cerebral vessels we used a commercial swept source optical coherence tomography system OCS1300SS (Thorlabs Inc. USA) operating at 1325 nm central wavelength and 100 nm bandwidth. Transverse and axial resolutions of OCT system are 25 µm and 12 µm respectively. A-scan rate is equal to 16 kHz, which allows us to measure absolute velocities up to ~5.5 mm/s [23,32].
The measurement of cerebral circulation was performed through the anterior fontanel in anesthetized rats (isoflurane – an inhalational anesthetic) with fixed head and scalp incision (the dura mater was left intact).
To quantify the changes in complexity of cerebral venous circulation (LSCI-data) we used the wavelet-based multifractal formalism [33] representing one of the most powerful approaches for statistical analysis of essentially nonstationary and inhomogeneous processes. It assumes computing the Hölder exponents (h) and the singularity spectrum D(h) according to algorithm proposed in [33] that consists of two stages. At the first stage, the wavelet transform of the analyzed signal is performed and the lines of local minima and maxima of the wavelet-transform coefficients are detected. At the second stage, the partition functions are computed and the singularity spectrum is estimated using the Legendre transform (see [33] for details). The range of Hölder exponent or the width of the singularity spectrum Δ = (hmax–hmin) is an informative measure of complexity of the signal. The simplest (the monofractal) signals are characterized by the measure Δ = 0. The values Δ>0 quantify more complicated dynamics, and the complexity increases with Δ.
Level of the blood oxygen saturation (SpO2) in the brain as an important criterion of cerebral metabolic activity in different functional states of organism [34] was monitored using pulse oximeter model CMS60D (Contec Medical Systems Co., Ltd., Qinhuangdao, China). Optical sensor was based on dual wavelengths pulse oximetry approach, using 660 nm and 880 nm for the SpO2 detection. The oxy-hemoglobin saturation (SpO2) is given as a percentage of HbO2 vs. the total Hb in the blood.
Beta-arrestin level was measured in the brain and blood serum in newborn rats by enzyme immunoassay (Beta-arrestin-1 (ARRB1) ELISA) according to standard protocol.
The results were reported as mean ± standard error of the mean (SEM). Differences from the initial level in the same group were evaluated by the Wilcoxon test. Intergroup differences were evaluated using the Mann-Whitney test and ANOVA-2 (post hoc analysis with the Duncan’s rank test). Significance levels were set at p < 0.05 for all analyses.
3. Results
3.1 Stress-related morphological changes in the brain tissues and the cerebral vessels in newborn rats
At the first step of our work, we analyzed the stress-induced changes in cerebral tissues and vessels associated with the development of NHS. With this aim, the brains of pups from the pre-stroke (4h after stress) and the post-stroke (24h after stress) groups underwent histological analysis. The results showed that cerebral veins are more sensitive to stress compared with cerebral arteries and vessels of microcirculatory bed. Indeed, the pre-stroke group compared with the control group demonstrated a congestion of excessive blood in the cerebral veins of the pia mater with an increased size of the veins (Fig. 1(a), 1(b)). These changes were associated with the perivascular edema in the cortex and subcortical tissues (Fig. 1(c)). The cerebral arteries and microvessels remained unchanged in this period of experiments. No intracranial hemorrhages were found 4h after stress in newborn rats.
Fig. 1.
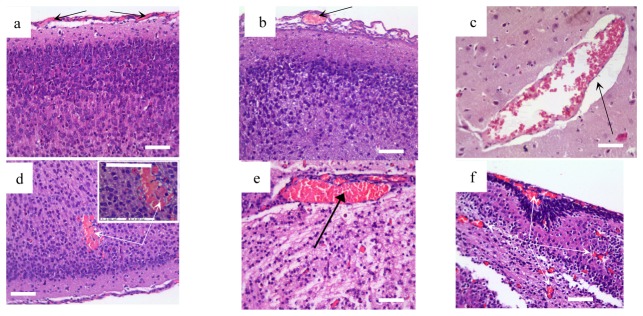
Cerebral vascular and parenchyma alterations induced by sound stress in newborn rats: a - the normal structure of vessels of the pia matter; b – congestion of excessive blood in the cerebral veins of the pia matter; c - perivascular edema; d - subcortical hemorrhage in low and high ( × 726.1) magnification; e - congestion of deep veins in the cerebral parenchyma; f - accumulation of blood in the microcirculatory vessels in the cortical and subcortical tissues. Hematoxylin & Eosin staining. Bars represent 10 µm ( × 246.4).
The stress-induced hemorrhages occurred in the next day after stress. Thus, 15 of 17 of newborn rats demonstrated small multifocal hemorrhages (average size 0.007 mm2 – 0.042 mm2) in the cortical and subcortical tissues (Fig. 1(d)). The mortality rate was 23% (4 of 17). The above-indicated venous abnormalities was progressed in the post-stroke group vs. the pre-stroke group. Venous congestion and cerebral edema were developed not only in the cortex but also in all regions of brain parenchyma that was associated with the accumulation of blood in microcirculatory bed (Fig. 1(f)).
Thus, the results of histological assay show that stress-reactivity of cerebral veins are higher than cerebral vessels in arterial and microcirculatory network. The dilation of cerebral veins and accumulation of blood in cerebral venous system precede the incidence of stroke while microcirculatory congestion occurs after the stroke.
3.2 Stress-related changes in the cerebral blood flow in newborn rats
At the second step of the study, we investigated the stress-induced changes in cerebral circulation at the level of venous and microcirculatory network using LSCI system and DOCT. The objects of the study were: 1) the sagittal sinus, which is one of major sinuses collecting blood from all veins of the brain and directs it into the peripheral circulation; 2) small, optically unresolvable vessels of microcirculatory bed surrounding the sagittal sinus. The choice of these regions was caused by a specificity of our approach to perform the monitoring of the CBF through the anterior fontanel as a window to the brain in newborns. The temporal dynamics of blood flow in investigated vessels (LCSI data) was analyzed by tracking the position of the most probable contrast value in the region of interest. As a reference, we choose a contrast value (which is inversely proportional to rCBF up to a constant) in the sagittal sinus in the control group. We set this value to unity and contrast values in other groups were recalculated as a fraction of this reference value.
LSCI results showed that the pre-stroke was associated with an increase in rCBF in the sagittal sinus but not in microcirculatory bed compared with the control group (Fig. 2). However, newborn rats with the stroke vs. healthy animals demonstrated an increased rCBF in both level of venous and microcirculatory components of cerebral circulation. Thus, rCBF changes in the sagittal sinus observed in all stages of stroke development, while the significant changes in microvessels occurred only in the post-stroke period.
Fig. 2.
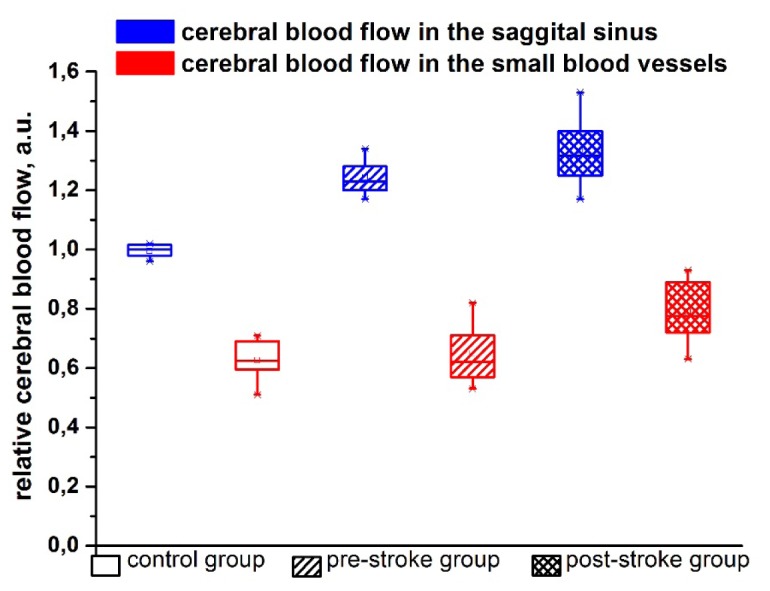
The relative cerebral blood flow assessed by LSCI in the sagittal sinus and in the small cerebral vessels (microcirculation) in newborn rats from the control, pre- and post-stroke groups.
DOCT imaging of the sagittal sinus showed the time-depended increase in diameter of this vessel with the fall of blood flow velocity. So, the diameter of the sagittal sinus was 1.7-fold and 3.7-fold higher in the pre- and post-stroke groups compared with control animals (0.51 ± 0.04 mm for the pre-stroke and 1.11 ± 0.02 mm for the post-stroke vs. 0.30 ± 0.03 mm for the control, p<0.05). The velocity of blood flow in the dilated sagittal sinus decreased by 48% (p<0.05) in the pre-stroke group, and by 65% (p<0.05) in the post-stroke group (3.11 ± 0.04 mm/sec for the pre-stroke and 2.09 ± 0.03 mm/sec for the post-stroke vs. 6.00 ± 0.09 mm/sec for the control, p<0.05).
Thus, LSCI results demonstrate that the stroke-induced changes are more pronounced in venous component of cerebral circulation compared with microcirculatory bed. Both LSCI and DOCT imaging clearly show the time-depended injures in the sagittal sinus such as the progressed vasorelaxation with suppression of blood flow velocity (DOCT) and an increased rCBF (LSCI).
Obvious conflict between DOCT and LSCI measurements can be overcome by the analysis of complexity of LSCI signal. Bries et al. clearly manifested the inequality of LSCI and DOCT signals relative to perfusion [35], since LSCI signal is affected by velocity of scatterers and their concentration. Further investigations of Kamzi et al. have demonstrated that a traditional LSCI system measures not flux or speed, but the product of RBC velocity and vessel diameter [36]. Recently Kosar et al. have quantified LSCI signal on optical properties of tissues [37]. If we account for all these results, we can avoid confusion between DOCT and LSCI. DOCT measurements confirmed vasodilation in pre- and post- stroke groups. As a result, there are much more RBCs within the vessel lumen in comparison to the normal group. Even if the speed of these cells is reduced (DOCT measurements), the rise of their concentration contributes significantly to the speckle contrast elevation showing the increase in rCBF index. A rough estimation of the RBC speed also can be done from LSCI measurements by dividing measured rCBF index by the corresponding vessel diameter D. For example, in the pre-stroke group ‘corrected’ index rCBF/D is equal to 0.65, which is smaller than for normal individuals (for control group rCBF/D index was set to unity). For the post-stroke group, the decrease of rCBF/D-ratio up to 0.36 was also found. Therefore, LSCI data are in a qualitative agreement with DOCT results.
3.3 Stress-related changes in a complexity of venous cerebral blood flow in newborn rats
In our previous work, we showed that the reduced microcirculatory variability is associated with stress-induced hemorrhages in stomach [38]. Reduced physiological complexity is often interpreted as a marker of cardiovascular [39–41] and neurological [42] “catastrophes.” We assumed that changes in the complexity of CBF can be an important characteristic the stress-reactivity of cerebral hemodynamics in newborn rats. Since histological and optical data showed more pronounced stress-induced changes in cerebral venous system, at the third step of the study we analyzed stress-related fluctuations in the venous CBF by the measure Δ reflecting complexity of CBF at different stages of stroke in newborn rats.
Figure 3 shows changes of the measure Δ for the pre-stroke and the post-stroke groups. An insert illustrates typical singularity spectra characterizing the dynamics of the venous CBF. It is clearly seen that the width of D(h), i.e., the complexity measure Δ is reduced at the development of pathological changes in the sagittal sinus. This effect is clearer expressed for the pre-stroke group as compared with the control group (0.86 ± 0.02 vs. 0.99 ± 0.04, p<0.05) that is important for early diagnostics of the high risk for the stroke. The post-stroke group is characterized by the value Δ = 0.90 ± 0.03, p<0.05. A reduced complexity of the venous CBF can be interpreted as a more homogeneous structure of LSCI data.
Fig. 3.
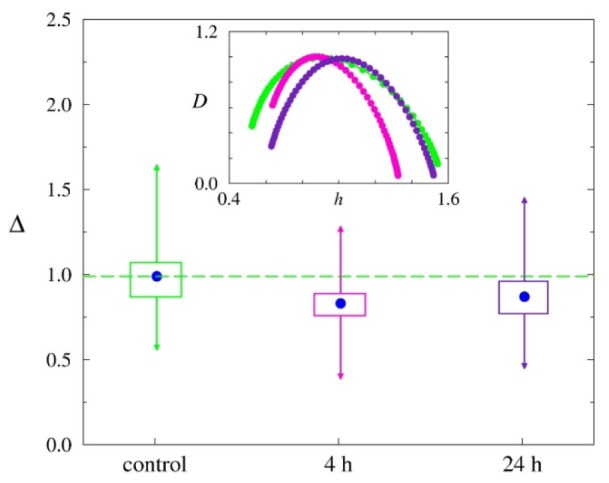
Stress-related changes in the complexity measure Δ representing the width of the singularity spectrum D(h) of the venous CBF in newborn rats from the control, pre- and post-stroke groups.
Thus, our results demonstrate that the stress-related changes in cerebral venous dynamics in newborn rats in the pre- and post-stroke groups is associated with a decrease in the complexity of venous CBF.
3.4 The stress-related changes in blood oxygen saturation of the brain tissues and in beta-arrestin-1 levels in the brain and blood in newborn rats
At the next step of the study, we focused on the determination of the stress-induced SpO2 changes in the brain of newborn rats as a main risk factor for the cerebrovascular catastrophes [30]. The results showed that the stress-related changes in the CBF was associated with reduced of SpO2 in the brain. The following SpO2 levels averaged for all animals were obtained: 98.1 ± 0.3% in the control group; 90.6 ± 1.8% (p<0.05) in the pre-stroke group; 76.5 ± 3.0% (p<0.05) in the post-stroke group.
At the final step of the experiments, we analyzed a relationship between stress-related hypoxia and the production of beta-arrestin-1 as an important factor of adrenergic regulation of vascular resistance to stress [29]. All stressed newborn rats demonstrated the elevation of beta-arrestin-1 level in the brain and in the blood compared with the control group. These stress-induced changes were more pronounced in the blood than in the brain and in the post-stroke group than in the pre-stroke group. So, beta-arrestin-1 level in the brain and blood was 1.9 fold and 2.5 fold higher in the pre-stroke group and 2.3 fold and 4.5 fold higher in the post-stroke group compared with the control group, respectively (Fig. 4).
Fig. 4.
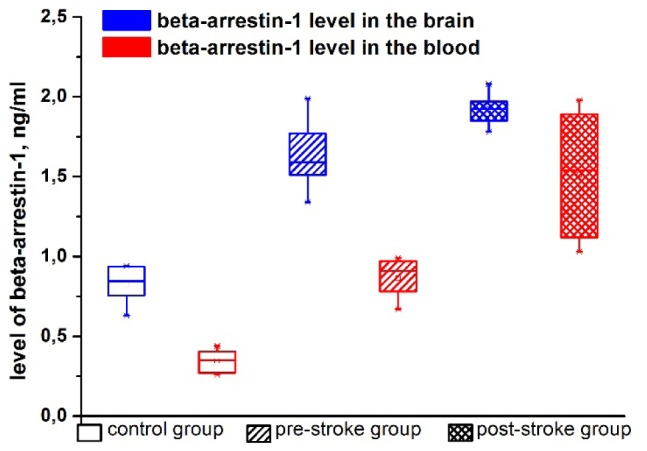
The stress-related changes in beta-arrestin-1 levels in the brain.
The results of this series of experiment show the time-depended changes in SpO2 in the brain and in the production of beta-arrestin-1 in the cerebral tissues and blood. The pre-stroke stage is characterized by mild hypoxia and increased synthesis of beta-arrestin-1, the post-stroke period is accompanied by progression of these pathological changes.
4. Discussion
Here we studied the stress-induced changes in the brain tissues and vessels associated with the stroke development using the optical assessment of CBF and SpO2 in the brain, histological evaluation of pathological changes in the cerebral tissues, a measure of complexity of CBF and level of beta-arrestin-1 as the markers of stress-resistance.
Our histological data show that the pre-stroke stage is associated with the increase in size of cerebral veins due to accumulation of extensive blood in them in superficial areas of the brain. These changes are associated with formation of perivascular edema, i.e. fluid pathway from the vessels. The relaxation of cerebral veins with perivascular edema are markers of accumulation of extensive blood in venous system and suppression of blood outflow from the brain leading to venous insufficiency [43]. The post-stroke time is associated with the progression of initial pathological changes in the cerebral venous system and the increasing of size of deep veins in the brain parenchyma that also was accompanied by accumulation of blood in microcirculatory bed. Thus, histological analysis suggest the venous vessels are more sensitive to harmful effect of stress than microvessels. In clinical studies also have been shown that neonatal stroke is primary venous infarction due to a weakness of the wall of cerebral veins in neonates [5,47,48].
The LSCI and DOCT results positively correlate with histological data. Indeed, LSCI results show the increase in rCBF in venous component of cerebral circulation in the pre-stroke stage and, in especially, in the post-stroke time. These results are consistent with clinical data suggesting that hyperperfusion is one of the stroke-associated damages of the brain [44–46]. Both LSCI and DOCT imaging show the time-depended progressive changes in the sagittal sinus from the initial stage of stroke until the incidence of brain bleeding.
Separate LSCI analysis of venous and microcirculatory components of cerebral circulation shows that the changes in the sagittal sinus occur earlier (in the pre-stroke stage) and are more pronounced than in microcirculatory network where the significant stress-response was only in the post-stroke time. We assume that the increase in rCBF in microvessels is the consequence of congestion of blood in the cerebral veins and the decreasing blood outflow from the brain. Therefore, the progressive relaxation of cerebral veins in newborn rats with the stroke causes accumulation of blood not only in venous network but also in microvessels due to the redistribution of blood flow in the cerebral vessels to decrease the pressure of accumulated blood on the think walls of cerebral veins.
Thus, histological and optical results suggest that accumulation of blood in dilated cerebral veins with the suppression of blood outflow from the brain due to decrease in the velocity of blood flow in cerebral venous system is a one of mechanisms underlying stress-induced brain injures associated with the stroke.
The hypoxia is one of main reasons for critical venous pathological changes associated with hemorrhages. Our results suggest that stroke-related venous relaxation and increased rCBF are accompanied by reduced SpO2 in the brain tissues. The relaxing effect of hypoxia on the brain vasculature and hypoxic-hyperperfusion is described also in other studies [49,50]. A normal physiological response to reduction of oxygen delivery is relaxation of cerebral vessels that activates metabolism in the brain tissues via the increasing of CBF. The particularities of cerebral veins are no muscles and valves in their thin walls, therefore, they have low resistance to critical stretching occurring during blood accumulation in them [51]. The immature brain vessels of newborns have limitation in vasorelaxation capabilities (increase of vessel size). The hypoxia-induced vasorelaxation of cerebral veins causes increasing of cerebral venous pressure [52]. This high pressure can induce easily the rupture of thin walls of immature cerebral veins of newborns [48].
Hypoxia causes the vascular stress due to critical relaxation of cerebral vessels via modulating of synthesis of beta-arrestin [29]. The beta-arresin is marker of stress-reactivity of vessels and vascular homeostasis [53]. We found the increased synthesis of this vascular stress factor in the brain and blood of newborn rats in all stages of stroke development. The level of beta-arrestin positively correlates with severity of stroke-related brain injuries. Thus, the activation of higher expression of beta-arrestin reflects the increase in stress-response intensity in the brain and peripheral circulatory system associated with the stroke-related changes in the cerebral tissues and vessels.
The inter-systemic marker of stress-resistance is the physiological complexity. Our results show the loss of normal complexity of the venous CBF in newborn rats in the initial and late stages of stroke. These data are consistent with clinical observations. So, a reduced complexity of the heart rate is associated with mortality in patients with head and torso trauma [54,55]. Complexity of heartbeat variability predicts an outcome in intensive care unit admitted patients with an acute stroke [56]. A decrease in physiological complexity is often interpreted as a marker of cardiovascular [39–41] and neurological [42] “catastrophes.” In our previous investigations on humans and animals, we showed that a reduced blood pressure and microcirculatory variability are associated with decreased stress-resistance of circulatory system [38,57].
5. Conclusion
In general, our results suggest that the stress-induced changes in cerebral veins are informative platform for the future study of mechanisms underlying NHS and development of effective strategy for prevention of stress-related intracranial hemorrhages during early life. Development of venous insufficiency with decrease in blood outflow from the brain that accompanied by hypoxia, reduction of complexity of venous blood flow and high production of beta-arrestin-1 are possible mechanisms responsible for a risk of neonatal hemorrhagic stroke.
Acknowledgments
O. S.-G., A. P., O. S., E. Z., M. U., and V. T. acknowledge support by Grant of Russian Science Foundation Nº 14-15-00128. E. B. and A. G. acknowledge support by Grant DFNI-B02/9/2014 of Bulgarian National Science Fund. D. Z. and Q. L. acknowledge support by National Nature Science Foundation of China (Grants No.81171376, 91232710), the Science Fund for Creative Research Group of China (Grant No.61421064), and the Programme of Introducing Talents of Discipline to Universities in China (Grant No. B07038).
References and links
- 1.Jordan L. C., Hillis A. E., “Hemorrhagic stroke in children,” Pediatr. Neurol. 36(2), 73–80 (2007). 10.1016/j.pediatrneurol.2006.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellekjaer H., Holmen J., Indredavik B., Terent A., “Epidemiology of stroke in Innherred, Norway, 1994 to 1996. Incidence and 30-day case-fatality rate,” Stroke 28(11), 2180–2184 (1997). 10.1161/01.STR.28.11.2180 [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Escusol S., Medrano-Marina P., Galván-Mansó M., Marco-Tello A., López-Pisón J., Rebage-Moisés V., “Focal cerebral ischemic or hemorrhagic lesions in the term newborn. Review of the last decade,” Rev. Neurol. 32(9), 801–805 (2001). [PubMed] [Google Scholar]
- 4.Gupta S. N., Kechli A. M., Kanamalla U. S., “Intracranial hemorrhage in term newborns: management and outcomes,” Pediatr. Neurol. 40(1), 1–12 (2009). 10.1016/j.pediatrneurol.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 5.Bruno C. J., Beslow L. A., Witmer C. M., Vossough A., Jordan L. C., Zelonis S., Licht D. J., Ichord R. N., Smith S. E., “Haemorrhagic stroke in term and late preterm neonates,” Arch. Dis. Child. Fetal Neonatal Ed. 99(1), F48–F53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takenouchi T., Kasdorf E., Engel M., Grunebaum A., Perlman J. M., “Changing pattern of perinatal brain injury in term infants in recent years,” Pediatr. Neurol. 46(2), 106–110 (2012). 10.1016/j.pediatrneurol.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 7.Wilson-Costello D., Friedman H., Minich N., Fanaroff A. A., Hack M., “Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s,” Pediatrics 115(4), 997–1003 (2005). 10.1542/peds.2004-0221 [DOI] [PubMed] [Google Scholar]
- 8.Whitby E. H., Griffiths P. D., Rutter S., Smith M. F., Sprigg A., Ohadike P., Davies N. P., Rigby A. S., Paley M. N., “Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors,” Lancet 363(9412), 846–851 (2004). 10.1016/S0140-6736(04)15730-9 [DOI] [PubMed] [Google Scholar]
- 9.Looney C. B., Smith J. K., Merck L. H., Wolfe H. M., Chescheir N. C., Hamer R. M., Gilmore J. H., “Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors,” Radiology 242(2), 535–541 (2007). 10.1148/radiol.2422060133 [DOI] [PubMed] [Google Scholar]
- 10.Lynch J. K., Han C. J., “Pediatric stroke: what do we know and what do we need to know?” Semin. Neurol. 25(4), 410–423 (2005). 10.1055/s-2005-923535 [DOI] [PubMed] [Google Scholar]
- 11.Brouwer A. J., Groenendaal F., Koopman C., Nievelstein R. J., Han S. K., de Vries L. S., “Intracranial hemorrhage in full-term newborns: a hospital-based cohort study,” Neuroradiology 52(6), 567–576 (2010). 10.1007/s00234-010-0698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirton A., Deveber G., “Life after perinatal stroke,” Stroke 44(11), 3265–3271 (2013). 10.1161/STROKEAHA.113.000739 [DOI] [PubMed] [Google Scholar]
- 13.Vohr B. R., Allan W. C., Westerveld M., Schneider K. C., Katz K. H., Makuch R. W., Ment L. R., “School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial,” Pediatrics 111(4), e340–e346 (2003). 10.1542/peds.111.4.e340 [DOI] [PubMed] [Google Scholar]
- 14.Fullerton H. J., Wu Y. W., Zhao S., Johnston S. C., “Risk of stroke in children: ethnic and gender disparities,” Neurology 61(2), 189–194 (2003). 10.1212/01.WNL.0000078894.79866.95 [DOI] [PubMed] [Google Scholar]
- 15.Towner D., Castro M. A., Eby-Wilkens E., Gilbert W. M., “Effect of mode of delivery in nulliparous women on neonatal intracranial injury,” N. Engl. J. Med. 341(23), 1709–1714 (1999). 10.1056/NEJM199912023412301 [DOI] [PubMed] [Google Scholar]
- 16.Benedetti T. J., “Birth injury and method of delivery,” N. Engl. J. Med. 341(23), 1758–1759 (1999). 10.1056/NEJM199912023412308 [DOI] [PubMed] [Google Scholar]
- 17.Lawn J. E., Kerber K., Enweronu-Laryea C., Cousens S., “3.6 million neonatal deaths - what is progressing and what is not?” Semin. Perinatol. 34(6), 371–386 (2010). 10.1053/j.semperi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 18.Paul V. K., “The current state of newborn health in low income countries and the way forward,” Semin. Fetal Neonatal Med. 11(1), 7–14 (2006). 10.1016/j.siny.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Craft T. K., Zhang N., Glasper E. R., Hurn P. D., Devries A. C., “Neonatal factors influence adult stroke outcome,” Psychoneuroendocrinology 31(5), 601–613 (2006). 10.1016/j.psyneuen.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 20.Manaenko A., Chen H., Zhang J. H., Tang J., “Comparison of different preclinical models of intracerebral hemorrhage,” Acta Neurochir. Suppl. (Wien) 111, 9–14 (2011). 10.1007/978-3-7091-0693-8_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLellan C. L., Paquette R., Colbourne F., “A critical appraisal of experimental intracerebral hemorrhage research,” J. Cereb. Blood Flow Metab. 32(4), 612–627 (2012). 10.1038/jcbfm.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlov A. N., Semyachkina-Glushkovskaya O. V., Zhang Y., Bibikova O. A., Pavlova O. N., Huang Q., Zhu D., Li P., Tuchin V. V., Luo Q., “Multiresolution analysis of pathological changes in cerebral venous dynamics in newborn mice with intracranial hemorrhage: adrenorelated vasorelaxation,” Physiol. Meas. 35(10), 1983–1999 (2014). 10.1088/0967-3334/35/10/1983 [DOI] [PubMed] [Google Scholar]
- 23.Semyachkina-Glushkovskaya O., Lychagov V., Bibikova O., Semyachkin-Glushkovskiy I., Sindeev S., Zinchenko E., Kassim M., Braun H., Al-Fatle F., Al Hassani L., Tuchin V., “The experimental study of stress-related pathological changes in cerebral venous blood flow in newborn rats assessed by DOCT,” J. Inn. Opt. Health Sci. 3(3), 1350023 (2013). [Google Scholar]
- 24.Thorburn R. J., Lipscomb A. P., Stewart A. L., Reynolds E. O. R., Hope P. L., “Timing and antecedents of periventricular haemorrhage and of cerebral atrophy in very preterm infants,” Early Hum. Dev. 7(3), 221–238 (1982). 10.1016/0378-3782(82)90085-8 [DOI] [PubMed] [Google Scholar]
- 25.Bassan H., Benson C. B., Limperopoulos C., Feldman H. A., Ringer S. A., Veracruz E., Stewart J. E., Soul J. S., Disalvo D. N., Volpe J. J., du Plessis A. J., “Ultrasonographic features and severity scoring of periventricular hemorrhagic infarction in relation to risk factors and outcome,” Pediatrics 117(6), 2111–2118 (2006). 10.1542/peds.2005-1570 [DOI] [PubMed] [Google Scholar]
- 26.Ballabh P., “Intraventricular hemorrhage in premature infants: mechanism of disease,” Pediatr. Res. 67(1), 1–8 (2010). 10.1203/PDR.0b013e3181c1b176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perlman J. M., McMenamin J. B., Volpe J. J., “Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage,” N. Engl. J. Med. 309(4), 204–209 (1983). 10.1056/NEJM198307283090402 [DOI] [PubMed] [Google Scholar]
- 28.Van Bel F., Van de Bor M., Stijnen T., Baan J., Ruys J. H., “Aetiological rôle of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage,” Dev. Med. Child Neurol. 29(5), 601–614 (1987). [DOI] [PubMed] [Google Scholar]
- 29.Lombardi M. S., van den Tweel E., Kavelaars A., Groenendaal F., van Bel F., Heijnen C. J., “Hypoxia/ischemia modulates G protein-coupled receptor kinase 2 and β-arrestin-1 levels in the neonatal rat brain,” Stroke 35(4), 981–986 (2004). 10.1161/01.STR.0000121644.82596.7e [DOI] [PubMed] [Google Scholar]
- 30.Michoulas A., Basheer S. N., Roland E. H., Poskitt K., Miller S., Hill A., “The role of hypoxia-ischemia in term newborns with arterial stroke,” Pediatr. Neurol. 44(4), 254–258 (2011). 10.1016/j.pediatrneurol.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 31.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies, Guide for the care and use of laboratory animals. 8th edition. Washington: The National Academies Press; 2011. http://oacu.od.nih.gov/regs/guide/guide.pdf. [Accessed 28 Feb 2012].
- 32.You J., Du C., Volkow N. D., Pan Y., “Optical coherence Doppler tomography for quantitative cerebral blood flow imaging,” Biomed. Opt. Express 5(9), 3217–3230 (2014). 10.1364/BOE.5.003217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muzy J. F., Bacry E., Arneodo A., “Wavelets and multifractal formalism for singular signals: Application to turbulence data,” Phys. Rev. Lett. 67(25), 3515–3518 (1991). 10.1103/PhysRevLett.67.3515 [DOI] [PubMed] [Google Scholar]
- 34.Liu N., Cui X., Bryant D. M., Glover G. H., Reiss A. L., “Inferring deep-brain activity from cortical activity using functional near-infrared spectroscopy,” Biomed. Opt. Express 6(3), 1074–1089 (2015). 10.1364/BOE.6.001074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briers D., Duncan D. D., Hirst E., Kirkpatrick S. J., Larsson M., Steenbergen W., Stromberg T., Thompson O. B., “Laser speckle contrast imaging: theoretical and practical limitations,” J. Biomed. Opt. 18(6), 066018 (2013). 10.1117/1.JBO.18.6.066018 [DOI] [PubMed] [Google Scholar]
- 36.Kazmi S. M., Faraji E., Davis M. A., Huang Y. Y., Zhang X. J., Dunn A. K., “Flux or speed? Examining speckle contrast imaging of vascular flows,” Biomed. Opt. Express 6(7), 2588–2608 (2015). 10.1364/BOE.6.002588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosar K., Kirkpatrick S. J., “Effects of combined scattering and absorption coefficients on laser speckle contrast imaging values”, Proc. SPIE 9322, 93220U (2015). [Google Scholar]
- 38.Pavlov A., Semyachkina-Glushkovskaya O., Pavlova O., Bibikova O., Kurths J., “Wavelet-analysis of gastric microcirculation in rats with ulcer bleedings,” Eur. Phys. J. Spec. Top. 222(10), 2705–2712 (2013). 10.1140/epjst/e2013-02050-7 [DOI] [Google Scholar]
- 39.Goldberger A. L., “Is the normal heartbeat chaotic or homeostatic?” News Physiol. Sci. 6, 87–91 (1991). [DOI] [PubMed] [Google Scholar]
- 40.Skinner J. E., Goldberger A. L., Mayer-Kress G., Ideker R. E., “Chaos in the heart: implication for clinical cardiology,” Nat. Biotechnol. 8(11), 1018–1024 (1990). 10.1038/nbt1190-1018 [DOI] [Google Scholar]
- 41.Goldberger A. L., Rigney D. R., Mietus J., Antman E. M., Greenwald S., “Nonlinear dynamics in sudden cardiac death syndrome: heartrate oscillations and bifurcations,” Experientia 44(11-12), 983–987 (1988). 10.1007/BF01939894 [DOI] [PubMed] [Google Scholar]
- 42.Babloyantz A., Destexhe A., “Low-dimensional chaos in an instance of epilepsy,” Proc. Natl. Acad. Sci. U.S.A. 83(10), 3513–3517 (1986). 10.1073/pnas.83.10.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdueza J. M., Doepp F., Schreiber S. J., van Oosten B. W., Schmierer K., Paul F., Wattjes M. P., “What went wrong? The flawed concept of cerebrospinal venous insufficiency,” J. Cereb. Blood Flow Metab. 33(5), 657–668 (2013). 10.1038/jcbfm.2013.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alderliesten T., Lemmers P. M., Smarius J. J., van de Vosse R. E., Baerts W., van Bel F., “Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage,” J. Pediatr. 162(4), 698–704 (2013). 10.1016/j.jpeds.2012.09.038 [DOI] [PubMed] [Google Scholar]
- 45.Taylor G. A., “New concepts in the pathogenesis of germinal matrix intraparenchymal hemorrhage in premature infants,” AJNR Am. J. Neuroradiol. 18(2), 231–232 (1997). [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor G. A., Trescher W. H., Johnston M. V., Traysman R. J., “Experimental neural injury in the newborn lamb: a comparison of NMDA receptor blockade and nitric oxide synthesis inhibition on lesion size and cerebral hyperemia,” Pediatr. Res. 38, 644–651 (1995). 10.1203/00006450-199511000-00003 [DOI] [PubMed] [Google Scholar]
- 47.Ghazi-Birry H. S., Brown W. R., Moody D. M., Challa V. R., Block S. M., Reboussin D. M., “Human germinal matrix: venous origin of hemorrhage and vascular characteristics,” AJNR Am. J. Neuroradiol. 18(2), 219–229 (1997). [PMC free article] [PubMed] [Google Scholar]
- 48.Hambleton G., Wigglesworth J. S., “Origin of intraventricular haemorrhage in the preterm infant,” Arch. Dis. Child. 51(9), 651–659 (1976). 10.1136/adc.51.9.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodin P., Burnstock G., “Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells,” Experientia 51(3), 256–259 (1995). 10.1007/BF01931108 [DOI] [PubMed] [Google Scholar]
- 50.Tomiyama Y., Brian J. E., Jr, Todd M. M., Pearce W., “Cerebral blood flow during hemodilution and hypoxia in rats : role of ATP-sensitive potassium channels,” Stroke 30(9), 1942–1948 (1999). 10.1161/01.STR.30.9.1942 [DOI] [PubMed] [Google Scholar]
- 51.Kiliç T., Akakin A., “Anatomy of cerebral veins and sinuses,” Front Neurol. Neurosci. 23, 4–15 (2007). 10.1159/000111256 [DOI] [PubMed] [Google Scholar]
- 52.Volpe J., “Intracranial hemorrhage: Germinal matrix hemorrhage”, In: Neurology of the Newborn. 5th. Philadelphia, PA: Saunders Elsevier; 403–463 (2008) [Google Scholar]
- 53.Hara M. R., Kovacs J. J., Whalen E. J., Rajagopal S., Strachan R. T., Grant W., Towers A. J., Williams B., Lam C. M., Xiao K., Shenoy S. K., Gregory S. G., Ahn S., Duckett D. R., Lefkowitz R. J., “A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1,” Nature 477(7364), 349–353 (2011). 10.1038/nature10368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riordan W. P., Jr, Norris P. R., Jenkins J. M., Morris J. A., Jr., “Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients,” J. Surg. Res. 156(2), 283–289 (2009). 10.1016/j.jss.2009.03.086 [DOI] [PubMed] [Google Scholar]
- 55.Norris P. R., Stein P. K., Morris J. A., Jr., “Reduced heart rate multiscale entropy predicts death in critical illness: a study of physiologic complexity in 285 trauma patients,” J. Crit. Care 23(3), 399–405 (2008). 10.1016/j.jcrc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 56.Tang S. C., Jen H. I., Lin Y. H., Hung C. S., Jou W. J., Huang P. W., Shieh J. S., Ho Y. L., Lai D. M., Wu A. Y., Jeng J. S., Chen M. F., “Complexity of heart rate variability predicts outcome in intensive care unit admitted patients with acute stroke,” J. Neurol. Neurosurg. Psychiatry 86(1), 95–100 (2015). 10.1136/jnnp-2014-308389 [DOI] [PubMed] [Google Scholar]
- 57.Anishchenko T., Igosheva N., Yakusheva T., Glushkovskaya-Semyachkina O., Khokhlova O., “Normalized entropy applied to the analysis of interindividual and gender-related differences in the cardiovascular effects of stress,” Eur. J. Appl. Physiol. 85(3-4), 287–298 (2001). 10.1007/s004210100470 [DOI] [PubMed] [Google Scholar]


