Figure 9.
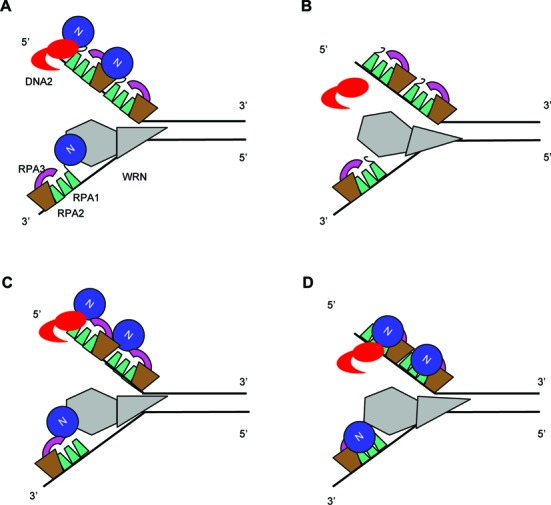
Model of the position of RPA1N and stimulation of WRN's 3′->5′ helicase activity and DNA2's 5′->3′ exonuclease activity. (A) RPA1N in the wildtype complex is optimally positioned to interact with WRN at the ss/ds-DNA junction on the 3′ strand and with DNA2 approaching from the 5′ end. RPA binding to the rest of ss-DNA prevents the reannealing of the unwound strands and the endonuclytic cleavage by DNA2. (B) 1NΔ RPA can still bind to ss-DNA to protect it against endonuclytic cleavage by DNA2 and to facilitate unwinding by preventing reannealing of the single strands unwound by the low intrinsic helicase activity of WRN. (C) RPA1N in the 3–1N RPA complex is still positioned close enough to interact normally with WRN and DNA2 and stimulate their activities. (D) RPA1N in the 1N-2 RPA complex is shifted more drastically in space and is less effective to recruit WRN to the ss/ds-DNA junction. It is completely incapable of recruiting DNA2 to the 5′ end of ss-DNA.
