Figure 2.
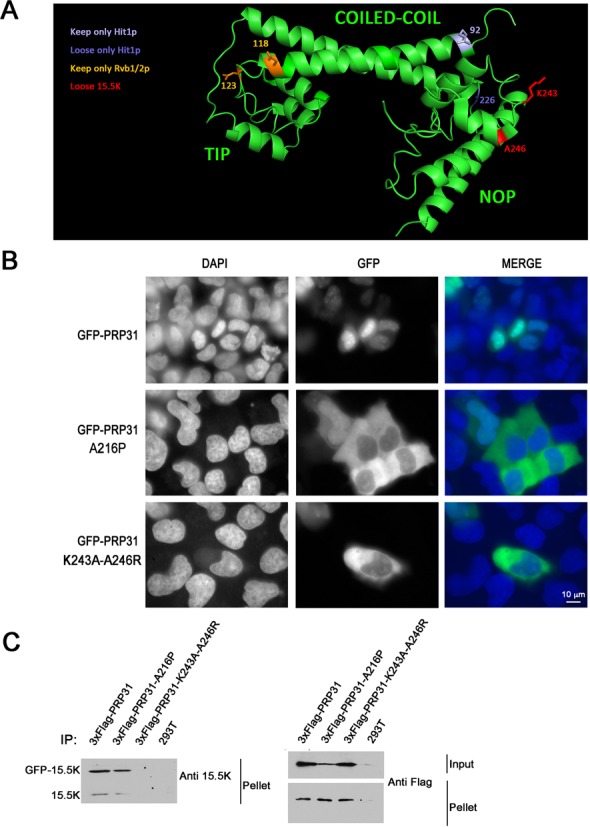
PRP31 A216P and PRP31 K243A-A246R are mostly cytoplasmic in human cells. (A) 3D Structure of human PRP31 with various mutations indicated. In yellow, amino-acids corresponding to MuA insertion sites leading to the loss of interaction with Pih1, Rsa1 and Hit1 (numbers correspond to the yeast protein). In blue, amino-acids corresponding to MuA insertion sites related to Hit1 (numbers correspond to the yeast protein). In red, amino acids mutated to prevent binding of 15.5K (numbers correspond to the human protein). (B) Micrographs showing the localization of wild-type and mutant PRP31 fused to GFP in U2OS cells. Scale bar is 10 μm. Blue: DAPI staining corresponding to nuclei; green: GFP-tagged PRP31 proteins. (C) PRP31 K243A-A246R does not interact with 15.5K in human cells. Western blotting of inputs and pellets of anti-Flag immuno-precipitates of 293T cells co-transfected with 3xFlag-PRP31 (wild-type and indicated mutants), GFP-15.5K or untransfected (293T). Western blots were probed with the indicated antibodies. Input: 5% of pellet.
