Figure 3.
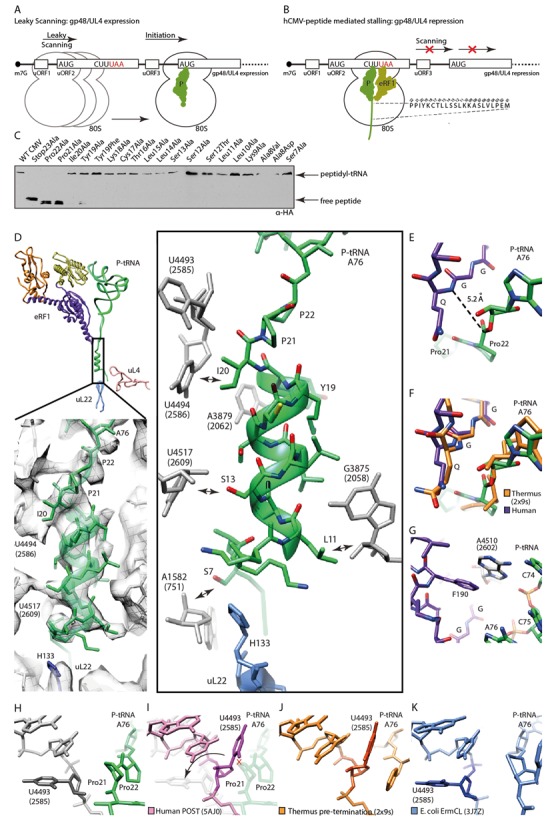
Mechanism of termination silencing by nascent hCMV peptide. (A) Schematic representation of the gp48/UL4 mRNA illustrating expression regulation of gp48/UL4 by leaky scanning of uORF2. (B) Ribosome stalling on the hCMV uORF2 leads to gp48/UL4 repression. (C) Identification of arrest-defective amino acid substitutions within the hCMV stalling peptide indicated by decreased amount of hCMV peptidyl-tRNA and accumulation of free peptide detected by Western blotting. (D, upper left) Overview of eRF1, peptidyl-tRNA and ribosomal proteins uL22 and uL4 labelled and coloured distinctively. (D, lower left) EM density section revealing the helical structure which is formed by the nascent hCMV peptide within the upper part of the ribosomal tunnel. (D, right) Same section, showing H133 of uL22 and nucleotides of the ribosomal tunnel wall which interact with the helical part of the nascent chain (arrows). (E) Position of the eRF1 GGQ-motif and the peptidyl-tRNA within the PTCs. (F) Comparison of the position of the bacterial RF2 and the human eRF1 GGQ-motifs and the peptidyl-tRNAs within the PTC. (G) eRF1-stabilizing position of Hs A4510 (Ec A2602). (H-K) Comparison of the position of Hs U4493 (Ec U2585) in (H) the hCMV stalled ribosome complex, (I) the human ribosome in the POST state, (J) the prokaryotic pre-termination complex and (K) the ErmCL stalled ribosomal complex.
