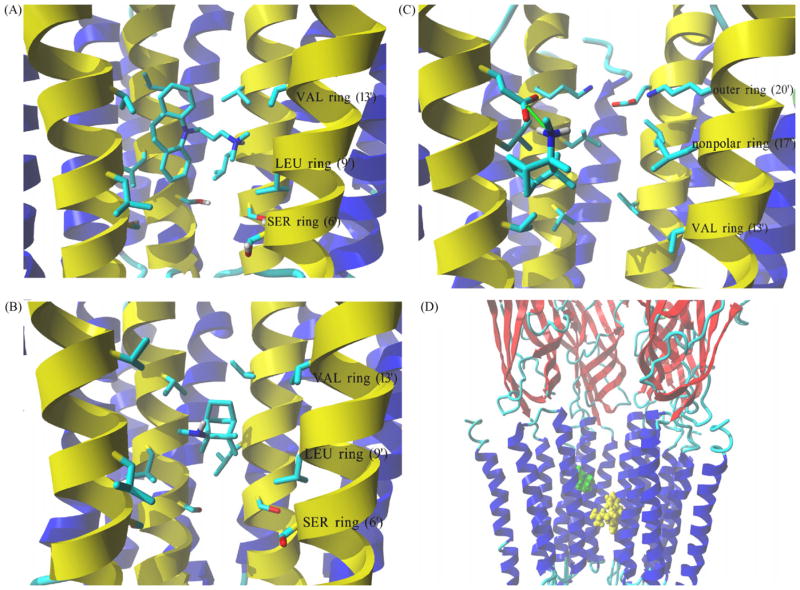
Fig. 8.
Complex formed between imipramine and S(+)-mecamylamine with the hα4β2 AChR ion channel model obtained by molecular docking. (A) Interaction of the protonated imipramine molecule with the leucine (LEU) (position 9′) and valine (VAL) (position 13′) rings in the hα4β2 AChR ion channel. Imipramine interacts with the LEU and VAL rings mainly by van der Waals contacts. (B) Interaction of neutral S(+)-mecamylamine with the LEU (position 9′) and VAL (position 13′) rings in the hα4β2 AChR ion channel. S(+)-Mecamylamine interacts with the LEU and VAL rings mainly by van der Waals forces. (C) Interaction of protonated S(+)-mecamylamine with the non-polar (position 17′) and outer (position 20′) rings in the hα4β2 AChR ion channel. Green arrow indicates coulombic interactions between positively charged amino groups from the ligand and a negatively charged carboxylic group from an α4-Glu261 residue. The aliphatic portion of S(+)-mecamylamine interacts with nearby Leu residues forming the non-polar ring (position 17′). (A–C) M2 transmembrane helices forming the wall of the channel are colored yellow, all other transmembrane segments are blue. Residues from each ring are shown explicitly in stick mode. Ligand molecules are rendered in element color coded stick mode. All non-polar hydrogen atoms are hidden. (D) Side view of the lowest energy complex formed between imipramine (yellow) and S(+)-mecamylamine (green), both in the protonated state, with the hα4β2 AChR ion channel. The imipramine binding site (located between LEU and VAL rings) is clearly distinct from that found for protonated S(+)-mecamylamine (located closer to the outer ring). Receptor subunits are shown in secondary structure mode (helices, blue; β-sheets, red), and ligands are shown in ball mode. Part of the receptor extracellular portion is also shown to have a better perspective of the ligand binding site locations. For clarity one β2 subunit is hidden thus, the order of explicitly shown subunits is (from left): β2, α4, β2 and α4. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)