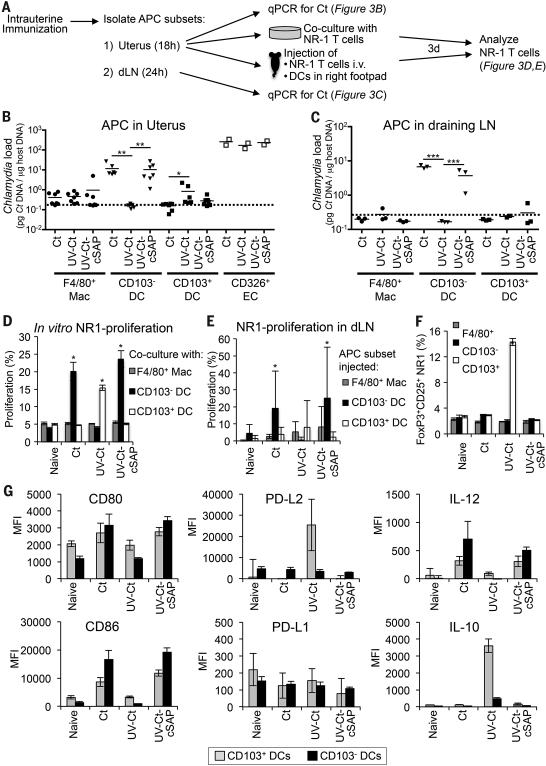
Figure 5. Distinct uterine DC subsets acquire Ags after i.u. Ct and UV-Ct-cSAP versus UV-Ct immunization and induce differential responses by Ct-specific T cells in vitro and in vivo.
(A) Schematic diagram of the experimental protocol for panels B-F. Mice were immunized i.u. with Ct, UV-Ct, or UV-Ct–cSAP. At indicated timepoints thereafter, CD45+MHC-II+ APC subsets were isolated from uteri and LNs and FACS sorted based on CD103 and F4/80 expression. Uptake of Ct per 1,000 sorted APCs in the uterus (B) and draining LN (C) was measured by qPCR. Isolated uterine CD326+ epithelial cells (EC) served as positive control for uterine samples. Data are pooled from two independent experiments. Mac, macrophages; DC, dendritic cells. n=2-7; broken line, limit of detection; *P<0.05; **P<0.01; ***P<0.001. (D) In vitro proliferation of NR1 TN was determined by CFSE dilution after incubation with sorted APC subsets for 3 days (n=4 mice/group; *P<0.05). (E) In vivo proliferation of CFSE-labeled CD90.1+ NR1 cells in a draining popliteal LN 3 days after footpad injection of APC subsets (n=4 mice/group; *P<0.05). (F) FoxP3-eGFP-depleted NR1 cells were incubated in vitro with sorted APC subsets. Frequencies of FoxP3-eGFP+ Treg were determined by flow cytometry after 3 days (n=4 mice/group; *P<0.05). (G) 18 hours after immunization uterine DC subsets were analyzed by FACS for indicated markers (n=4). Error bars represent mean ± SEM. Statistical differences were assessed using one-way ANOVA followed by Bonferroni's post-test.