Abstract
Background
Utilization of primary care may decrease colorectal cancer (CRC) incidence and death through greater receipt of CRC screening tests.
Objective
To examine the association of primary care utilization with CRC incidence, CRC deaths, and all-cause mortality.
Design
Population-based, case–control study.
Setting
Medicare program.
Participants
Persons aged 67 to 85 years diagnosed with CRC between 1994 and 2005 in U.S. Surveillance, Epidemiology, and End Results (SEER) regions matched with control patients (n = 205 804 for CRC incidence, 54 160 for CRC mortality, and 121 070 for all-cause mortality).
Measurements
Primary care visits in the 4- to 27-month period before CRC diagnosis, CRC incidence, CRC mortality, and all-cause mortality.
Results
Compared with persons having 0 or 1 primary care visit, persons with 5 to 10 visits had lower CRC incidence (adjusted odds ratio [OR], 0.94 [95% CI, 0.91 to 0.96]) and mortality (adjusted OR, 0.78 [CI, 0.75 to 0.82]) and lower all-cause mortality (adjusted OR, 0.79 [CI, 0.76 to 0.82]). Associations were stronger in patients with late-stage CRC diagnosis, distal lesions, and diagnosis in more recent years when there was greater Medicare screening coverage. Ever receipt of CRC screening and polypectomy mediated the association of primary care utilization with CRC incidence.
Limitation
This study used administrative data, which made it difficult to identify potential confounders and prevented examination of the content of primary care visits.
Conclusion
Medicare beneficiaries with higher utilization of primary care have lower CRC incidence and mortality and lower overall mortality. Increasing and promoting access to primary care in the United States for Medicare beneficiaries may help decrease the national burden of CRC.
Primary Funding Source
American Cancer Society.
In the United States, colorectal cancer (CRC) is the source of much morbidity and mortality and increased health care costs. In 2013, there will be an estimated 142 820 new cases and 50 830 deaths from CRC (1). Five-year costs to Medicare for care of persons diagnosed with CRC in a single year exceed $3 billion (2). Colorectal cancer may be prevented through screening tests and polypectomy (3). Yet, only 59% of U.S. adults aged 50 years or older have ever received CRC screening (4), and merely 39% of CRC cases are diagnosed at a localized stage (5).
Primary care plays an important role in increasing CRC screening rates because the recommendation of a primary care physician (PCP) is one of the strongest predictors of adherence to CRC screening (6, 7). Areas with more PCPs have been previously found to have lower CRC incidence (8, 9) and mortality (10). However, those studies were limited by the ecologic fallacy that whether persons with better outcomes are the same ones who visited primary care cannot be determined. Our previous cohort study of Medicare beneficiaries with CRC, which described the association between PCP visits with early-stage diagnosis and reduced CRC mortality (11), was subject to lead-time bias because it did not have a comparison group. Therefore, the improved survival observed with primary care visits could have been due to earlier diagnosis without actual changes in mortality. In addition, that study could not examine CRC incidence.
The anticipated shortage of PCPs over the next decade in the United States (12, 13), coupled with the lack of PCP use by some Medicare beneficiaries (11, 14), may create barriers to achieving national goals of reducing CRC incidence and mortality. (15). In this study, we extend our prior work by examining the association of utilization of PCPs with CRC incidence and actual mortality within a population-based sample. We hypothesized that Medicare beneficiaries with few or no visits to a PCP would have higher CRC incidence and mortality, mediated by ever receipt of previous CRC screening and polypectomy.
Methods
Using a retrospective case–control study design, we examined the relationship between PCP visits and CRC incidence, CRC-specific mortality, and all-cause mortality in the 2008 Surveillance, Epidemiology and End Results (SEER)–Medicare linked data set (16). This study was approved by the Institutional Review Boards of the University of South Florida and Beth Israel Deaconess Medical Center.
Study Sample
Inclusion and Exclusion Criteria
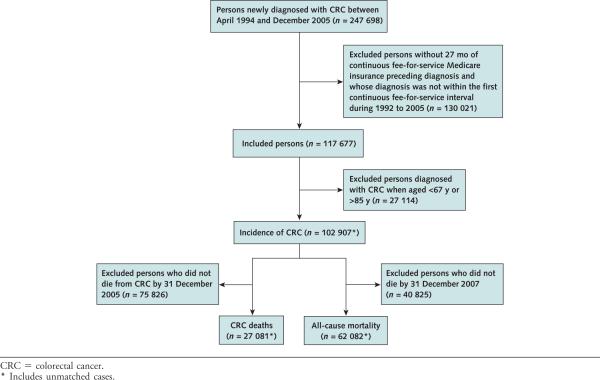
Persons newly diagnosed with CRC within the SEER program between April 1994 and December 2005 represented cases of interest (n = 247 698). We excluded cases without 27 months of continuous fee-for-service Medicare insurance preceding diagnosis and whose diagnosis was not within their first continuous fee-for-service interval during 1992 to 2005 (n = 117 677). Because eligibility for Medicare usually begins at age 65 years and CRC screening is not recommended for persons older than 85 years (3), we excluded individuals (n = 27 114) diagnosed before age 67 years or after age 85 years, yielding a final incidence case number of 102 907. For the CRC-specific mortality analysis, those who did not die of CRC by 31 December 2005 (n = 75 826) were excluded, yielding a final case number of 27 081. For the all-cause mortality analysis, we excluded incident case participants who did not die by 31 December 2007 based on Medicare death year (n = 40 825) for a final case number of 62 082 (Appendix Figure, available at www.annals.org).
The control participants, representing the population at risk, were selected similarly to cases from the 5% random sample of persons residing in the SEER regions. Those within the 5% random sample who did not have CRC were eligible control participants for the incidence analysis (n = 485 350). Persons within the 5% random sample both with and without CRC who did not die of CRC by 31 December 2005 were eligible as control participants in the CRC-specific mortality analysis (n = 489 238). For the all-cause mortality analysis, eligible control participants were those within the 5% random sample who did not die by 31 December 2007 (n = 303 120).
Matching Criteria
Case patients were matched to control participants 1:1 using a greedy algorithm (17, 18). Each case patient was matched to 1 control participant who had the same original reason for Medicare entitlement (age, disability, end-stage renal disease), lived in the same SEER area as the case patient during the year of the case's diagnosis, had his or her birth year within 5 years of the case patient's birth year, and had at least 27 months of continuous fee-for-service Medicare enrollment before the month of the case patient's diagnosis. We did not match on sex to assess its potential risk factor on our outcomes. We successfully matched approximately 100% of incidence (final n = 102 902) and CRC-specific mortality cases (final n = 27 080) and 97.5% of all-cause mortality cases (final n = 60 535) used for our analyses.
Utilization of Primary Care
We examined Medicare claims for ambulatory-based evaluation and management services representing routine office visits (Current Procedural Terminology codes 99201–99205, 99211–99215) (19) and identified the physician specialty associated with each claim using the Medicare provider specialty field (20). The PCPs were defined as general practice, family medicine, primary care internal medicine, geriatric medicine, and obstetrics–gynecology. We included obstetrics–gynecology as primary care because 64.3% of visits to these providers are for routine follow-up or preventive care (21) and they made up only 2% of claims. All other specialties were defined as non-PCPs.
For each participant, we calculated the total number of ambulatory claims to PCPs during the 4- to 27-month period before diagnosis. Because physician visit patterns are likely to change while a potential case of cancer is being diagnosed, we excluded the 3-month period immediately before diagnosis (22). We created categories of PCP visits corresponding to quartiles 0 to 1, 2 to 4, 5 to 10, or 11 or more visits because distribution of the number of visits was very skewed. Visits to non-PCPs were assessed similarly.
Cancer Screening and Polypectomy
We used Medicare claims history to assess ever receipt of CRC screening and polypectomy procedures up to 10 years before diagnosis. Screening tests were defined as any claim with Current Procedural Terminology (23); International Classification of Diseases, Ninth Revision (19); and Healthcare Common Procedure Coding System (24) codes, as previously described (11). We examined the claims for the case and control participants in each matched pair for the same interval of time (mean, 6.74 [SD, 2.68]; median, 6.92 [interquartile range, 4.25 to 9.58]). We excluded screening or polypectomy claims submitted in the 3 months immediately preceding CRC diagnosis to remove procedures potentially related to the diagnosis (22, 25, 26).
Statistical Analysis
We examined the relationship between the number of PCP visits (4 to 27 months before diagnosis) and CRC incidence, CRC-specific mortality, and all-cause mortality using conditional logistic regression, conditioning on matched pairs. Potential confounders in multivariable models included number of non-PCP visits, sex, race or ethnicity (reported by Medicare), census-derived measures of median household income and educational attainment at the ZIP code level (categorized by quintiles within each registry), metropolitan statistical area, Charlson comorbidity index (determined through inpatient and outpatient claims) (27), and influenza vaccination in the previous 4 to 27 months (as a marker of healthy behaviors in persons seeking more primary care) (28). Because the effect of PCP visits on CRC incidence and death may be mediated by CRC screening and polypectomy, the multivariable models were fit without and then with these variables. We examined our multivariate models for multicollinearity among the covariates and found it to be negligible. Based on a study by Lin and colleagues (29), we also examined the impact of an unmeasured confounder on our estimated odds ratios (ORs) in post hoc sensitivity analyses.
Colorectal cancer screening may preferentially reduce cases of late-stage and distal cancer (11). In addition, Medicare coverage of CRC screening has changed over time. We therefore conducted stratified analyses to investigate these potential effect modifiers. The cancer stage at diagnosis was classified using the American Joint Committee on Cancer staging system, with early-stage CRC defined as 0 and I and late stages as II, III, and IV (30, 31). Cancer of the cecum, appendix, ascending colon, hepatic flexure, transverse colon, and splenic flexure was considered proximal; cancer of the descending colon, sigmoid colon, rectosigmoid junction, and rectum was considered distal (11, 32). Years of diagnosis were divided by Medicare's coverage of screening procedures: no coverage from 1994 to 1997, limited coverage between 1998 and 2000 (yearly fecal occult blood test, flexible sigmoidoscopy every 4 years, colonoscopy only for high-risk persons), and full coverage after 2000 (colonoscopy every 10 years for all persons). All analyses were performed using SAS, version 9.3 (SAS Institute, Cary, North Carolina), and R software, version 2.15.2. All tests of statistical significance were 2-sided.
Role of the Funding Source
This study was funded by the American Cancer Society, which had no role in the study design, collection, analysis, interpretation, or drafting of the manuscript or in the decision to submit the manuscript for publication.
Results
The mean age of our sample was 76.2 years (SD, 5.1) and 76.7 years (SD, 5.2) for CRC incidence and mortality cohorts, respectively (Appendix Table 1, available at www.annals.org). Number of visits to a PCP for the incidence study population was similar between case and control participants (mean, 7.0 vs. 7.3; median, 5.0 vs. 5.0, respectively). In addition, 21.5% of incident case participants had no visits to a PCP versus 20.1% of incident control participants. When compared with cases, a higher percentage of incident control participants had ever received CRC screening (45.8% case participants vs. 52.6% control participants) or polypectomy (13.9% case participants vs. 18.1% control participants). The mean age of those who received CRC screening was 76.8 years (SD, 4.9; median, 77 years [interquartile range, 73 to 81 years]), whereas the mean age of those who did not was 75.7 years (SD, 5.3; median, 76 years [interquartile range, 71 to 80 years]). For the CRC-mortality study population, there was a greater difference between mortality case and control participants in the proportion having 0 or 1 PCP visit and receipt of previous CRC screening and polypectomy. The mean age of those who received screening in the CRC-mortality study population was 77.3 years (SD, 5.0; median, 78 years [interquartile range, 73 to 81 years]), whereas the mean age of those who did not was 76.2 years (SD, 5.3; median, 76 years [interquartile range, 72 to 81 years]). In addition, 25.6% of case participants who died of CRC had no visits to a PCP versus 20.5% of control participants. Moreover, 14% of mortality case patients who died of CRC and 10% of mortality control patients did not have visits to a PCP or non-PCP.
CRC Incidence
The likelihood of CRC diagnosis decreased with increasing primary care visits (Table 1). Compared with persons having 0 to 1 primary care visits, those who had 5 to 10 visits had 6% lower odds of CRC diagnosis (model 1). When receipt of previous screening or polypectomy was added (model 2), the association of PCP visits and CRC diagnosis disappeared. In stratified analyses, associations of PCP visits with lower CRC incidence were found in patients with late-stage diagnosis and distal lesions as well as in those diagnosed during years with greater Medicare coverage of CRC screening tests (Figure 1). There was a higher likelihood of diagnosis of early-stage cancer and proximal cancer with increased primary care visits.
Table 1.
Predictors of CRC Incidence*
| Variable | Unadjusted OR (95% CI) | Multivariable Model 1† OR (95% CI) | Multivariable Model 2‡ OR (95% CI) |
|---|---|---|---|
| Total ambulatory physician visits to PCP | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–4 | 0.94 (0.92–0.97) | 0.98 (0.95–1.01) | 1.03 (1.00–1.05) |
| 5–10 | 0.90 (0.88–0.92) | 0.94 (0.91–0.96) | 1.00 (0.98–1.03) |
| ≥11 | 0.89 (0.87–0.91) | 0.93 (0.90–0.95) | 1.00 (0.97–1.03) |
| Total ambulatory physician visits to non-PCP | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–4 | 0.98 (0.96–1.00) | 0.98 (0.95–1.00) | 1.01 (0.98–1.03) |
| 5–10 | 0.97 (0.94–0.99) | 0.94 (0.92–0.97) | 0.98 (0.96–1.01) |
| ≥11 | 0.98 (0.96–1.01) | 0.92 (0.89–0.94) | 0.98 (0.96–1.01) |
| Sex | |||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Female | 0.69 (0.68–0.70) | 0.69 (0.67–0.70) | 0.69 (0.68–0.70) |
| Race/ethnicity | |||
| White, non-Hispanic | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Black, non-Hispanic | 1.18 (1.14–1.22) | 1.13 (1.09–1.18) | 1.13 (1.09–1.17) |
| Hispanic | 0.61 (0.57–0.66) | 0.57 (0.53–0.62) | 0.56 (0.52–0.60) |
| Asian or Pacific Islander | 0.75 (0.71–0.80) | 0.70 (0.66–0.74) | 0.68 (0.65–0.72) |
| Native American | 0.70 (0.59–0.83) | 0.70 (0.58–0.83) | 0.68 (0.57–0.82) |
| Other | 0.93 (0.87–1.00) | 0.88 (0.82–0.95) | 0.88 (0.81–0.94) |
| Unknown | 0.81 (0.65–1.00) | 0.78 (0.63–0.98) | 0.77 (0.62–0.97) |
| Quintile of education level (derived at ZIP code level) | |||
| 1 (lowest) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 0.98 (0.95–1.01) | 0.92 (0.89–0.95) | 0.92 (0.90–0.95) |
| 3 | 0.98 (0.96–1.01) | 0.90 (0.87–0.93) | 0.91 (0.88–0.94) |
| 4 | 0.93 (0.91–0.96) | 0.82 (0.79–0.85) | 0.84 (0.81–0.87) |
| 5 (highest) | 0.83 (0.81–0.86) | 0.72 (0.69–0.75) | 0.74 (0.71–0.77) |
| Quintile of median household income (derived at ZIP code level) | |||
| 1 (lowest) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 1.04 (1.01–1.07) | 1.11 (1.07–1.14) | 1.11 (1.07–1.14) |
| 3 | 1.05 (1.03–1.08) | 1.15 (1.11–1.19) | 1.15 (1.12–1.19) |
| 4 | 1.02 (0.99–1.05) | 1.18 (1.13–1.22) | 1.18 (1.13–1.22) |
| 5 (highest) | 0.95 (0.93–0.98) | 1.21 (1.16–1.26) | 1.21 (1.16–1.26) |
| Type of metropolitan statistical area | |||
| Large metropolitan | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Metropolitan | 0.90 (0.88–0.93) | 0.91 (0.88–0.94) | 0.91 (0.88–0.93) |
| Urban | 0.93 (0.89–0.96) | 0.91 (0.87–0.95) | 0.91 (0.87–0.95) |
| Less urban | 0.90 (0.86–0.93) | 0.88 (0.84–0.92) | 0.87 (0.83–0.91) |
| Rural | 0.86 (0.80–0.92) | 0.84 (0.78–0.90) | 0.83 (0.77–0.89) |
| Charlson comorbidity index | |||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 | 1.09 (1.06–1.11) | 1.12 (1.09–1.14) | 1.11 (1.09–1.14) |
| ≥2 | 1.17 (1.14–1.20) | 1.19 (1.16–1.22) | 1.18 (1.15–1.21) |
| Influenza vaccination | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.93 (0.91–0.94) | 0.95 (0.93–0.96) | 0.97 (0.95–0.99) |
| Previous CRC screening | |||
| No | 1.00 (reference) | NA | 1.00 (reference) |
| Yes | 0.75 (0.74–0.77) | NA | 0.81 (0.79–0.83) |
| Previous polypectomy | |||
| No | 1.00 (reference) | NA | 1.00 (reference) |
| Yes | 0.72 (0.70–0.74) | NA | 0.80 (0.78–0.83) |
CRC = colorectal cancer; NA = not applicable; OR = odds ratio; PCP = primary care physician.
The total number of CRC case participants and matching control participants was 205 804.
Adjusted for primary care visits, non–primary care visits, sex, race/ethnicity, quintile of education level, quintile of median household income, urban or rural residence, comorbid conditions, and influenza vaccination.
Adjusted for primary care visits, non–primary care visits, sex, race/ethnicity, quintile of education level, quintile of median household income, urban or rural residence, comorbid conditions, influenza vaccination, history of CRC screening, and history of polypectomy.
Figure 1.
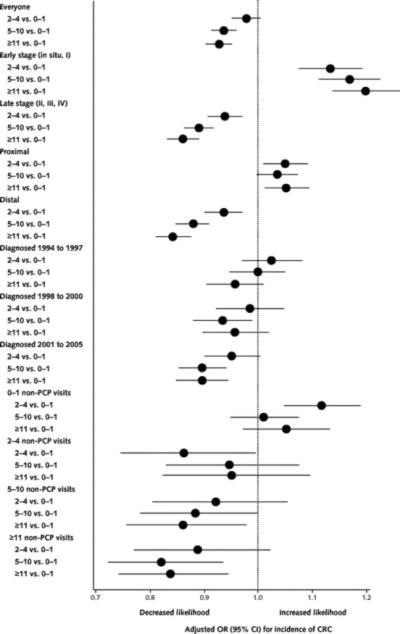
Association between number of PCP visits and CRC incidence, stratified by early- versus late-stage CRC, proximal versus distal CRC, year of CRC diagnosis, and number of non-PCP visits.
CRC-Specific and All-Cause Mortality
In multivariable analyses adjusting for all factors except previous CRC screening and polypectomy (model 1), persons with 5 to 10 PCP visits had 22% lower odds of CRC mortality than those with 0 or 1 visit (Table 2). In analyses including CRC screening and polypectomy (model 2), the relationship of PCP visits with fewer CRC deaths was slightly attenuated. In stratified analyses, associations of PCP visits with fewer CRC deaths were stronger for patients with late- versus early-stage diagnosis, those with distal versus proximal lesions, and those diagnosed during years with greater Medicare coverage of screening tests (Figure 2).
Table 2.
Predictors of CRC Death*
| Variable | Unadjusted OR (95% CI) | Multivariable Model 1† OR (95% CI) | Multivariable Model 2‡ OR (95% CI) |
|---|---|---|---|
| Total ambulatory physician visits to PCP | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–4 | 0.82 (0.78–0.87) | 0.88 (0.84–0.93) | 0.95 (0.90–1.00) |
| 5–10 | 0.72 (0.69–0.75) | 0.78 (0.75–0.82) | 0.86 (0.82–0.90) |
| ≥11 | 0.71 (0.68–0.75) | 0.80 (0.76–0.85) | 0.89 (0.85–0.95) |
| Total ambulatory physician visits to non-PCP | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–4 | 0.80 (0.76–0.83) | 0.84 (0.80–0.89) | 0.88 (0.84–0.92) |
| 5–10 | 0.74 (0.71–0.78) | 0.76 (0.73–0.80) | 0.81 (0.77–0.85) |
| ≥11 | 0.68 (0.65–0.72) | 0.67 (0.63–0.71) | 0.74 (0.70–0.78) |
| Sex | |||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Female | 0.70 (0.68–0.72) | 0.70 (0.67–0.72) | 0.70 (0.67–0.72) |
| Race/ethnicity | |||
| White, non-Hispanic | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Black, non-Hispanic | 1.39 (1.30–1.48) | 1.22 (1.13–1.31) | 1.21(1.12–1.30) |
| Hispanic | 0.56 (0.49–0.65) | 0.52 (0.45–0.60) | 0.51 (0.44–0.59) |
| Asian or Pacific Islander | 0.76 (0.68–0.85) | 0.69 (0.61–0.78) | 0.68 (0.60–0.77) |
| Native American | 0.73 (0.52–1.02) | 0.65 (0.45–0.93) | 0.61 (0.43–0.88) |
| Other | 1.05 (0.90–1.21) | 0.97 (0.83–1.13) | 0.93 (0.79–1.08) |
| Unknown | 1.00 (0.69–1.47) | 0.89 (0.60–1.32) | 0.85 (0.57–1.27) |
| Quintile of education level (derived at ZIP code level) | |||
| 1 (lowest) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 0.92 (0.87–0.97) | 0.89 (0.83–0.94) | 0.89 (0.84–0.95) |
| 3 | 0.91 (0.87–0.96) | 0.88 (0.82–0.93) | 0.89 (0.83–0.95) |
| 4 | 0.84 (0.80–0.89) | 0.79 (0.74–0.85) | 0.81 (0.75–0.87) |
| 5 (highest) | 0.76 (0.72–0.80) | 0.69 (0.64–0.75) | 0.73 (0.67–0.79) |
| Quintile of median household income (derived at ZIP code level) | |||
| 1 (lowest) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 1.01 (0.96–1.07) | 1.13 (1.06–1.20) | 1.13 (1.07–1.20) |
| 3 | 0.97 (0.92–1.03) | 1.12 (1.05–1.20) | 1.13 (1.05–1.20) |
| 4 | 0.93 (0.88–0.98) | 1.14 (1.06–1.23) | 1.14 (1.06–1.23) |
| 5 (highest) | 0.89 (0.84–0.94) | 1.21 (1.11–1.32) | 1.21 (1.11–1.32) |
| Type of metropolitan statistical area | |||
| Large metropolitan | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Metropolitan | 0.84 (0.77–0.91) | 0.80 (0.73–0.87) | 0.78 (0.72–0.86) |
| Urban | 0.85 (0.80–0.90) | 0.86 (0.81–0.91) | 0.86 (0.81–0.91) |
| Less urban | 0.78 (0.68–0.89) | 0.72 (0.63–0.83) | 0.71 (0.62–0.82) |
| Rural | 0.86 (0.79–0.93) | 0.83 (0.76–0.90) | 0.83 (0.76–0.91) |
| Charlson comorbidity index | |||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 | 0.98 (0.94–1.03) | 1.11 (1.06–1.17) | 1.11 (1.06–1.16) |
| ≥2 | 1.08 (1.03–1.13) | 1.23 (1.17–1.29) | 1.23 (1.16–1.29) |
| Influenza vaccination | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.76 (0.74–0.79) | 0.86 (0.83–0.90) | 0.89 (0.86–0.93) |
| Previous CRC screening | |||
| No | 1.00 (reference) | NA | 1.00 (reference) |
| Yes | 0.62 (0.60–0.64) | NA | 0.75 (0.72–0.78) |
| Previous polypectomy | |||
| No | 1.00 (reference) | NA | 1.00 (reference) |
| Yes | 0.55 (0.52–0.58) | NA | 0.70 (0.66–0.74) |
CRC = colorectal cancer; NA = not applicable; OR = odds ratio; PCP = primary care physician.
The total number of CRC deaths and matching control participants was 54 160.
Adjusted for primary care visits, non–primary care visits, sex, race/ethnicity, quintile of education level, quintile of median household income, urban or rural residence, comorbid conditions, and influenza vaccination.
Adjusted for primary care visits, non–primary care visits, sex, race/ethnicity, quintile of education level, quintile of median household income, urban or rural residence, comorbid conditions, influenza vaccination, history of CRC screening, and history of polypectomy.
Figure 2.
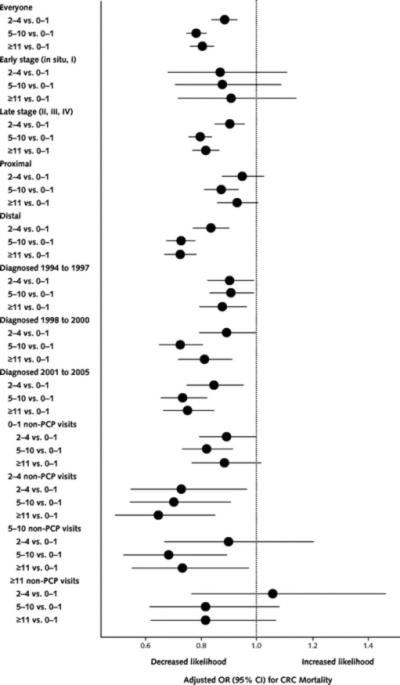
Association between number of PCP visits and CRC mortality, stratified by early- versus late-stage CRC, proximal versus distal CRC, year of CRC diagnosis, and number of non-PCP visits.
In analyses that controlled for all covariates except CRC screening and polypectomy, having at least 2 PCP visits was associated with approximately 20% lower odds of all-cause mortality compared with the reference group (adjusted OR, 0.79 to 0.82) (Appendix Table 2, available at www.annals.org). This association was attenuated but not eliminated when CRC screening and polypectomy were added (adjusted OR, 0.88 to 0.92). Excluding in situ lesions from the sample did not significantly alter results for all 3 analyses.
Effect of PCP Visits Stratified by Non-PCP Visits
A very low correlation existed between PCP and non-PCP utilization (Spearman rank correlation coefficient, 0.19, 0.21, and 0.19 for incidence, CRC mortality, and all-cause mortality, respectively; P < 0.001). Because non-PCP visits showed similar effects on outcomes as PCP visits, we reanalyzed each outcome stratified by categories of non-PCP visits to disentangle the effect of PCP visits from any physician visit (Figures 1 and 2). The association of PCP visits with lower CRC incidence and mortality generally persisted in persons having high numbers of non-PCP visits. For example, among patients having 5 to 10 non-PCP visits, those with 5 to 10 and 11 or more PCP visits had a 12% (adjusted OR, 0.88 [95% CI, 0.78 to 0.99]) and 14% (adjusted OR, 0.86 [CI, 0.76 to 0.98]) lower odds of CRC incidence and a 32% (adjusted OR, 0.68 [CI, 0.52 to 0.89]) and 27% (adjusted OR, 0.72 [CI, 0.55 to 0.97]) lower odds of CRC mortality, respectively, compared with patients with 0 to 1 PCP visit.
Discussion
Medicare beneficiaries with higher utilization of primary care had lower CRC incidence, CRC-specific mortality, and all-cause mortality. Ever receipt of CRC screening or polypectomy explained the association between primary care utilization and CRC incidence. However, more than 54% of incident case participants had no claim for any CRC screening in the previous 10 years. Utilization of non-PCPs was also associated with lower CRC incidence and mortality, implying that greater access to health care generally improves CRC outcomes. The effect of non-PCP visits may reflect the effect of PCP visits because these physicians facilitate referrals and access to specialists. Our stratified analyses by non-PCP visits showed that regardless of the number of non-PCP visits, utilization of PCPs conferred independent and additional benefits even on patients having many visits to non-PCPs.
Despite universal Medicare insurance, more than 20% of beneficiaries had no visits to a PCP over a 24-month period, and approximately 10% had no visits to any physician. These beneficiaries had higher CRC incidence, CRC mortality, and all-cause mortality. Medicare's recent expanded coverage for preventive care and annual wellness visits may help emphasize the importance of PCP visits and preventive screenings (33). However, the current difficul-ties in accessing primary care will be exacerbated by the looming primary care shortage along with the influx of newly insured adults using primary care because of recently enacted health reform law (12, 13, 34). Policies and programs are needed to increase access to and supply of PCPs.
This large population-based, case–control study is not subject to recall bias and overcomes limitations of previous ecologic studies (8–10) and the potential lead-time bias of our prior cohort study (11). In addition, our stratified analyses allowed us to explore some relevant and biologically plausible mechanisms for the associations we found. For example, the influence of primary care on lower CRC incidence was mediated by receipt of previous CRC screening and polypectomy. This is supported by the stronger associations of primary care with CRC outcomes during years with greater Medicare coverage for CRC screening. Moreover, we found preferential effects of PCP visits on incidence of and mortality from distal versus proximal CRC and late- versus early-stage cancer. This is consistent with previous studies showing that screening has beneficial effects on reducing distal but not proximal CRC incidence and mortality (35) and mortality in late- but not early-stage cancer (36).
The increased incidence of proximal cancer associated with PCP visits may be due to residual confounding by comorbidity because of its greater relation to proximal CRC (P < 0.001) (32) and subsequent higher PCP utilization. In addition, because CRC screening prevents distal cancer more than proximal cases, screening may be uncovering more occult lesions. Alternatively, findings might have been due to chance because CIs were close to the null.
Receipt of previous CRC screening or polypectomy attenuated but did not fully eliminate the association of PCP visits with lower CRC mortality. The explanation for this finding is uncertain, but several potential possibilities exist. For example, PCPs may encourage healthier behaviors (such as diet, exercise, weight control, not smoking, use of aspirin, avoidance of environmental carcinogens, and receipt of other preventive services) (37) and coordinate care to reduce medication and laboratory errors (38). Or PCPs may facilitate access to specialists, which improves management of comorbid illnesses present in many patients with cancer (39). Another possibility is the healthy-user effect, in which healthier patients are more likely to seek primary care and adhere to medications or use preventive services (28). We included influenza vaccination in our models as a proxy for healthy behavior; however, this may not fully account for the healthy-user effect. Further analyses with influenza vaccination removed from our model did not significantly change the effect of PCP visits or the effect of previous CRC screening on outcomes. Alternatively, we may not have identified some screening and polypectomy procedures because of coding or other errors. For example, because of our Medicare sample and truncated exposure history starting at age 65 years, CRC screening and polypectomy performed before that age were not captured in our data.
There are limitations to consider when interpreting our findings. First, this study included only persons aged 67 to 85 years having fee-for-service Medicare insurance who were predominantly white and living in SEER registry areas. Results may not be generalizable to Medicare beneficiaries enrolled in managed care plans. Second, it was also limited to administrative data contained within the SEER–Medicare database. Therefore, residual confounding by unmeasured factors may influence results. In post hoc sensitivity analyses, our results become nonsignificant or potentially reversed only when the proportions of an un-measured confounder differed greatly between individuals who visit PCPs often and those who do not. An unmeasured confounder seems to have a greater effect on the association of PCP with CRC incidence than with mortality (Appendix Tables 3 and 4, available at www.annals.org). Third, our measure of primary care was limited to number of visits, so we could not assess the content or quality of visits. Thus, it is uncertain what specific aspects of the primary care visit are beneficial in improving CRC outcomes. Finally, we did not differentiate between CRC screening versus diagnostic procedures, so the true rate of ever receipt of CRC screening may be lower than the 49% found. Inclusion of diagnostic tests could limit our ability to determine whether the effect of primary care utilization was mediated by receipt of screening. However, we excluded any CRC screening in the 3 months before diagnosis to remove procedures potentially related to CRC diagnosis. Further research is needed on how utilization of primary care affects CRC incidence and mortality and whether our results are reproducible in other populations and for other types of cancer.
This study complements and extends previous evidence of the benefits of primary care in improving health outcomes (40). In particular, primary care helps decrease CRC by promoting screening and facilitating referrals for colonoscopy and polypectomy. Because a recommendation from primary care is one of the strongest predictors of adherence to CRC screening (6, 7) and several different options are available for CRC screening, access to primary care is important for counseling on these options. The move toward patient-centered medical homes will probably improve rates of CRC screening because of the model's emphasis on preventive care and tracking of tests in its patient population. Promoting and increasing access to primary care for Medicare beneficiaries may help decrease the national burden of CRC.
See also:
Editorial comment. . . . . . . . . . . . . . . . . . . . . . . . . . 494
Summary for Patients. . . . . . . . . . . . . . . . . . . . . . . I-24
Context
Many people who could benefit are not screened for colorectal cancer.
Contribution
This study found that people enrolled in Medicare who had more primary care visits were less likely to develop or die from colorectal cancer and had a lower mortality from all causes combined.
Caution
This study used an observational design, which cannot establish cause and effect.
Implication
Improving access to primary care and encouraging people to use primary care may help decrease the national burden of colorectal cancer.
—The Editors
Acknowledgment
The authors thank the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services; and the SEER program tumor registries in the creation of the SEER–Medicare database.
Grant Support: By the American Cancer Society (grant RSGHP-08-141-01-CPHPS). This study used the linked SEER–Medicare database. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code, section 103885; the National Cancer Institute's SEER program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute.
Appendix Figure.
Study flow diagram.
Appendix Table 1.
Characteristics of Case and Control Participants*
| Variable | CRC Incidence, n (%)† |
CRC Deaths, n (%)‡ |
||
|---|---|---|---|---|
| Case Participants | Control Participants | Case Participants | Control Participants | |
| Total ambulatory visits to PCP | ||||
| 0–1 | 29 089 (28.3) | 27 179 (26.4) | 8942 (33.0) | 7289 (26.9) |
| 2–4 | 19 451 (18.9) | 19 246 (18.7) | 5129 (18.9) | 5068 (18.7) |
| 5–10 | 29 681 (28.8) | 30 677 (29.8) | 7146 (26.4) | 8066 (29.8) |
| ≥11 | 24 681 (24.0) | 25 800 (25.1) | 5863 (21.7) | 6657 (24.6) |
| Total ambulatory visits to non-PCP | ||||
| 0–1 | 40 622 (39.5) | 39 993 (38.9) | 12 540 (46.3) | 10 615 (39.2) |
| 2–4 | 21 164 (20.6) | 21 300 (20.7) | 5333 (19.7) | 5646 (20.9) |
| 5–10 | 22 047 (21.4) | 22 485 (21.9) | 5158 (19.1) | 5870 (21.7) |
| ≥11 | 19 069 (18.5) | 19 124 (18.6) | 4049 (15.0) | 4949 (18.3) |
| Age | ||||
| ≤70 y | 17 618 (17.1) | 17 710 (17.2) | 4113 (15.2) | 4142 (15.3) |
| 71–75 y | 28 604 (27.8) | 28 517 (27.7) | 7146 (26.4) | 7146 (26.4) |
| 76–80 y | 31 088 (30.2) | 31 092 (30.2) | 8248 (30.5) | 8196 (30.3) |
| ≥81 y | 25 592 (24.9) | 25 583 (24.9) | 7573 (28.0) | 7596 (28.1) |
| Sex | ||||
| Male | 50 153 (48.7) | 40 768 (39.6) | 13 018 (48.1) | 10 681 (39.4) |
| Female | 52 749 (51.3) | 62 134 (60.4) | 14 062 (51.9) | 16 399 (60.6) |
| Race/ethnicity | ||||
| White, non-Hispanic | 88 306 (85.8) | 87 898 (85.4) | 22 950 (84.8) | 23 188 (85.6) |
| Black, non-Hispanic | 8264 (8.0) | 7073 (6.9) | 2567 (9.5) | 1909 (7.1) |
| Hispanic | 1341 (1.3) | 2113 (2.1) | 343 (1.3) | 598 (2.2) |
| Asian or Pacific Islander | 2851 (2.8) | 3545 (3.5) | 653 (2.4) | 832 (3.1) |
| Native American | 229 (0.2) | 318 (0.3) | 63 (0.2) | 83 (0.3) |
| Other | 1757 (1.7) | 1766 (1.7) | 450 (1.7) | 416 (1.5) |
| Unknown | 154 (0.2) | 189 (0.2) | 54 (0.2) | 54 (0.2) |
| Quintile of education level (derived at ZIP code level) | ||||
| 1 (lowest) | 20 654 (20.2) | 19 068 (19.1) | 5999 (22.4) | 5216 (19.9) |
| 2 | 20 821 (20.4) | 19 552 (19.5) | 5531 (20.6) | 5227 (19.9) |
| 3 | 20 708 (20.3) | 19 374 (19.5) | 5564 (20.8) | 5284 (20.1) |
| 4 | 20 481 (20.1) | 20 204 (20.3) | 5100 (19.0) | 5268 (20.1) |
| 5 (highest) | 19 363 (19.0) | 21 395 (21.5) | 4604 (17.2) | 5258 (20.0) |
| Quintile of median household income (derived at ZIP code level) | ||||
| 1 (lowest) | 20 474 (20.1) | 20 269 (20.4) | 5689 (21.2) | 5358 (20.4) |
| 2 | 20 703 (20.3) | 19 632 (19.7) | 5560 (20.7) | 5179 (19.7) |
| 3 | 20 701 (20.3) | 19 436 (19.5) | 5414 (20.2) | 5252 (20.0) |
| 4 | 20 479 (20.1) | 19 882 (20.0) | 5278 (19.7) | 5329 (20.3) |
| 5 (highest) | 19 670 (19.3) | 20 374 (20.5) | 4857 (18.1) | 5135 (19.6) |
| Type of metropolitan statistical area | ||||
| Large metropolitan | 57 252 (55.6) | 55 915 (54.5) | 15 201 (56.1) | 14 688 (54.4) |
| Metropolitan | 27 658 (26.9) | 28 457 (27.7) | 7140 (26.4) | 7429 (27.5) |
| Urban | 6765 (6.6) | 6737 (6.6) | 1787 (6.6) | 1819 (6.7) |
| Less urban | 9136 (8.9) | 9311 (9.1) | 2399 (8.9) | 2466 (9.1) |
| Rural | 2087 (2.0) | 2224 (2.2) | 550 (2.0) | 607 (2.2) |
| Charlson comorbidity index | ||||
| 0 | 58 682 (57.0) | 61 480 (59.8) | 16 057 (59.3) | 16 204 (59.8) |
| 1 | 24 059 (23.4) | 23 257 (22.6) | 5938 (21.9) | 6095 (22.5) |
| ≥2 | 20 161 (19.6) | 18 165 (17.7) | 5085 (18.8) | 4781 (17.7) |
| Influenza vaccination | ||||
| No | 50 932 (49.5) | 48 994 (47.6) | 14 859 (54.9) | 13 071 (48.3) |
| Yes | 51 970 (50.5) | 53 908 (52.4) | 12 221 (45.1) | 14 009 (51.7) |
| Previous CRC screening | ||||
| No | 55 747 (54.2) | 48 767 (47.4) | 16 145 (59.6) | 13 044 (48.2) |
| Yes | 47 155 (45.8) | 54 135 (52.6) | 10 935 (40.4) | 14 036 (51.8) |
| Screening method§ | ||||
| Fecal occult blood test | 38 903 (82.5) | 44 809 (82.8) | 9002 (82.3) | 11 617 (82.8) |
| Flexible sigmoidoscopy | 2255 (4.8) | 2755 (5.1) | 478 (4.4) | 677 (4.8) |
| Barium enema | 9716 (20.6) | 10 970 (20.3) | 2475 (22.6) | 3094 (22.0) |
| Colonoscopy | 10 362 (22.0) | 14 938 (27.6) | 2024 (18.5) | 3582 (25.5) |
| Previous polypectomy | ||||
| No | 88 571 (86.1) | 84 268 (81.9) | 24 431 (90.2) | 22 710 (83.9) |
| Yes | 14 331 (13.9) | 18 634 (18.1) | 2649 (9.8) | 4370 (16.1) |
| Stage at diagnosis | ||||
| 0 (in situ) | 7449 (7.2) | NA | 229 (0.9) | NA |
| I | 20 696 (20.1) | NA | 1283 (4.7) | NA |
| II | 26 305 (25.6) | NA | 3980 (14.7) | NA |
| III | 21 337 (20.7) | NA | 6745 (24.9) | NA |
| IV | 15 066 (14.6) | NA | 10 748 (39.7) | NA |
CRC = colorectal cancer; NA = not applicable; PCP = primary care physician.
All case and control comparisons are significant at P < 0.001 except non-PCP visits (P = 0.021 for incidence comparisons), age (P = 0.80 for incidence comparisons and 0.89 for mortality comparisons), comorbid conditions (P = 0.002 for mortality comparisons), fecal occult blood test (P = 0.25 for incidence comparisons and 0.36 for mortality comparisons), barium enema (P = 0.180 for incidence comparisons and 0.27 for mortality comparisons), and flexible sigmoidoscopy (P = 0.025 for incidence comparisons and 0.092 for mortality comparisons).
The total number of CRC case participants and matching control participants was 205 804.
The total number of CRC deaths and matching control participants was 54 160.
Of those with previous CRC screening. Numbers do not add to total because some participants had more than 1 screening method.
Appendix Table 2.
Predictors of All-Cause Mortality*
| Variable | Unadjusted OR (95% CI) | Multivariable Model 1† OR (95% CI) | Multivariable Model 2‡ OR (95% CI) |
|---|---|---|---|
| Total ambulatory physician visits to PCP | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–4 | 0.75 (0.73–0.78) | 0.82 (0.79–0.85) | 0.88 (0.85–0.92) |
| 5–10 | 0.76 (0.74–0.79) | 0.79 (0.76–0.82) | 0.88 (0.84–0.91) |
| ≥11 | 0.90 (0.88–0.93) | 0.81 (0.78–0.85) | 0.92 (0.88–0.95) |
| Total ambulatory physician visits to non-PCP | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–4 | 0.85 (0.83–0.88) | 0.82 (0.79–0.85) | 0.86 (0.83–0.89) |
| 5–10 | 0.89 (0.86–0.91) | 0.78 (0.76–0.81) | 0.84 (0.81–0.87) |
| ≥11 | 1.11 (1.07–1.15) | 0.82 (0.79–0.86) | 0.91 (0.88–0.95) |
| Sex | |||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Female | 0.53 (0.52–0.54) | 0.53 (0.52–0.55) | 0.53 (0.52–0.55) |
| Race/ethnicity | |||
| White, non-Hispanic | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Black, non-Hispanic | 1.36 (1.30–1.42) | 1.14 (1.08–1.20) | 1.13 (1.07–1.19) |
| Hispanic | 0.57 (0.52–0.63) | 0.48 (0.43–0.53) | 0.46 (0.41–0.51) |
| Asian or Pacific Islander | 0.57 (0.53–0.61) | 0.47 (0.43–0.51) | 0.45 (0.42–0.49) |
| Native American | 0.85 (0.68–1.07) | 0.68 (0.53–0.87) | 0.64 (0.50–0.83) |
| Other | 0.86 (0.78–0.95) | 0.76 (0.68–0.84) | 0.75 (0.67–0.83) |
| Unknown | 0.98 (0.76–1.27) | 0.92 (0.70–1.23) | 0.91 (0.68–1.21) |
| Quintile of education level (derived at ZIP code level) | |||
| 1 (lowest) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 0.90 (0.87–0.93) | 0.88 (0.84–0.91) | 0.88 (0.84–0.92) |
| 3 | 0.89 (0.85–0.92) | 0.83 (0.79–0.87) | 0.85 (0.81–0.89) |
| 4 | 0.80 (0.77–0.83) | 0.74 (0.70–0.78) | 0.76 (0.72–0.80) |
| 5 (highest) | 0.67 (0.65–0.70) | 0.61 (0.58–0.65) | 0.64 (0.60–0.68) |
| Quintile of median household income (derived at ZIP code level) | |||
| 1 (lowest) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 0.97 (0.94–1.01) | 1.11 (1.07–1.16) | 1.12 (1.07–1.17) |
| 3 | 0.93 (0.90–0.97) | 1.12 (1.06–1.17) | 1.12 (1.07–1.17) |
| 4 | 0.92 (0.89–0.95) | 1.19 (1.13–1.25) | 1.18 (1.12–1.25) |
| 5 (highest) | 0.81 (0.78–0.84) | 1.21 (1.14–1.29) | 1.21 (1.14–1.29) |
| Type of metropolitan statistical area | |||
| Large metropolitan | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Metropolitan | 0.88 (0.84–0.91) | 0.87 (0.84–0.91) | 0.87 (0.83–0.91) |
| Urban | 0.93 (0.88–0.98) | 0.86 (0.81–0.91) | 0.86 (0.81–0.92) |
| Less urban | 0.87 (0.82–0.91) | 0.78 (0.74–0.83) | 0.78 (0.73–0.83) |
| Rural | 0.80 (0.73–0.88) | 0.70 (0.64–0.78) | 0.69 (0.63–0.76) |
| Charlson comorbidity index | |||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 | 1.61 (1.56–1.66) | 1.77 (1.72–1.83) | 1.76 (1.70–1.82) |
| ≥2 | 3.07 (2.96–3.17) | 3.36 (3.23–3.49) | 3.34 (3.21–3.47) |
| Influenza vaccination | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.83 (0.81–0.85) | 0.83 (0.81–0.85) | 0.87 (0.85–0.89) |
| Previous CRC screening | |||
| No | 1.00 (reference) | NA | 1.00 (reference) |
| Yes | 0.64 (0.62–0.65) | NA | 0.71 (0.69–0.73) |
| Previous polypectomy | |||
| No | 1.00 (reference) | NA | 1.00 (reference) |
| Yes | 0.60 (0.58–0.63) | NA | 0.72 (0.69–0.75) |
CRC = colorectal cancer; NA = not applicable; OR = odds ratio; PCP = primary care physician.
The total number of deaths from all causes matching control participants was 121 070.
Adjusted for primary care visits, non–primary care visits, sex, race/ethnicity, quintile of education level, quintile of median household income, urban or rural residence, comorbid conditions, and influenza vaccination.
Adjusted for primary care visits, non–primary care visits, sex, race/ethnicity, quintile of education level, quintile of median household income, urban or rural residence, comorbid conditions, influenza vaccination, history of CRC screening, and history of polypectomy.
Appendix Table 3.
Point Estimates and 95% CIs for the Odds of CRC Incidence Associated With 5 to 10 PCP Visits Versus 0 to 1 PCP Visit*
| OR† | P1‡ | P0§ |
|||||
|---|---|---|---|---|---|---|---|
| 0.05 | 0.1 | 0.2 | 0.3 | 0.5 | 0.8 | ||
| 0.9 | 0.05 | 0.94 (0.91–0.96) | – | – | – | – | – |
| 0.1 | 0.94 (0.91–0.96) | 0.94 (0.91–0.96) | – | – | – | – | |
| 0.2 | 0.95 (0.92–0.97) | 0.95 (0.92–0.97) | 0.94 (0.91–0.96) | – | – | – | |
| 0.3 | 0.96 (0.93–0.98) | 0.96 (0.93–0.98) | 0.95 (0.92–0.97) | 0.94 (0.91–0.96) | – | – | |
| 0.5 | 0.98 (0.95–1.01) | 0.98 (0.95–1.00) | 0.97 (0.94–0.99) | 0.96 (0.93–0.98) | 0.94 (0.91–0.96) | – | |
| 0.8 | 1.02 (0.98–1.04) | 1.01 (0.98–1.03) | 1.00 (0.97–1.02) | 0.99 (0.96–1.01) | 0.97 (0.94–0.99) | 0.94 (0.91–0.96) | |
| 0.75 | 0.05 | 0.94 (0.91–0.96) | – | – | – | – | – |
| 0.1 | 0.95 (0.92–0.97) | 0.94 (0.91–0.96) | – | – | – | – | |
| 0.2 | 0.98 (0.95–1.00) | 0.96 (0.93–0.99) | 0.94 (0.91–0.96) | – | – | – | |
| 0.3 | 1.00 (0.97–1.02) | 0.99 (0.96–1.01) | 0.97 (0.93–0.99) | 0.94 (0.91–0.96) | – | – | |
| 0.5 | 1.06 (1.03–1.08) | 1.05 (1.01–1.07) | 1.02 (0.99–1.04) | 0.99 (0.96–1.01) | 0.94 (0.91–0.96) | – | |
| 0.8 | 1.16 (1.12–1.18) | 1.15 (1.11–1.17) | 1.12 (1.08–1.14) | 1.09 (1.05–1.11) | 1.03 (1.00–1.05) | 0.94 (0.91–0.96) | |
CRC = colorectal cancer; OR = odds ratio; PCP = primary care physician.
With adjustment for an unmeasured confounder having an OR of CRC incidence of 0.9 or 0.75 (n = 205 804). Bold indicates that CIs include 1.0 (PCP effect becomes null) or are >1.0 (PCP effect is reversed).
OR of CRC incidence comparing persons with an unmeasured confounder vs. those without the confounder.
Prevalence of unmeasured confounder in those with 5 to 10 PCP visits.
Prevalence of unmeasured confounder in those with 0 to 1 PCP visit.
Appendix Table 4.
Point Estimates and 95% CIs for the Odds of CRC Deaths Associated With 5 to 10 PCP Visits Versus 0 to 1 PCP Visit*
| OR† | P1‡ | P0§ |
|||||
|---|---|---|---|---|---|---|---|
| 0.05 | 0.1 | 0.2 | 0.3 | 0.5 | 0.8 | ||
| 0.9 | 0.05 | 0.86 (0.82–0.90) | – | – | – | – | – |
| 0.1 | 0.86 (0.82–0.90) | 0.86 (0.82–0.90) | – | – | – | – | |
| 0.2 | 0.87 (0.83–0.91) | 0.87 (0.83–0.91) | 0.86 (0.82–0.90) | – | – | – | |
| 0.3 | 0.88 (0.84–0.92) | 0.88 (0.84–0.92) | 0.87 (0.83–0.91) | 0.86 (0.82–0.90) | – | – | |
| 0.5 | 0.90 (0.86–0.94) | 0.90 (0.85–0.94) | 0.89 (0.85–0.93) | 0.88 (0.84–0.92) | 0.86 (0.82–0.90) | – | |
| 0.8 | 0.93 (0.89–0.97) | 0.93 (0.88–0.97) | 0.92 (0.87–0.96) | 0.91 (0.86–0.95) | 0.89 (0.85–0.93) | 0.86 (0.82–0.90) | |
| 0.75 | 0.05 | 0.86 (0.82–0.90) | – | – | – | – | – |
| 0.1 | 0.87 (0.83–0.91) | 0.86 (0.82–0.90) | – | – | – | – | |
| 0.2 | 0.89 (0.85–0.94) | 0.883 (0.842–0.924) | 0.86 (0.82–0.9) | – | – | – | |
| 0.3 | 0.92 (0.88–0.96) | 0.91 (0.86–0.95) | 0.88 (0.84–0.92) | 0.86 (0.82–0.90) | – | – | |
| 0.5 | 0.97 (0.93–1.02) | 0.96 (0.91–1.00) | 0.93 (0.89–0.98) | 0.91 (0.87–0.95) | 0.86 (0.82–0.90) | – | |
| 0.8 | 1.06 (1.01–1.11) | 1.05 (1.05–1.10) | 1.02 (0.97–1.07) | 0.99 (0.95–1.04) | 0.94 (0.90–0.98) | 0.86 (0.82–0.90) | |
CRC = colorectal cancer; OR = odds ratio; PCP = primary care physician.
With adjustment for an unmeasured confounder having an OR of CRC death of 0.9 or 0.75 (n = 54 160). Bold indicates that CIs include 1.0 (PCP effect becomes null) or are >1.0 (PCP effect is reversed).
OR of CRC death comparing persons with an unmeasured confounder vs. those without the confounder.
Prevalence of unmeasured confounder in those with 5 to 10 PCP visits.
Prevalence of unmeasured confounder in those with 0 to 1 PCP visit.
Footnotes
Author Contributions: Conception and design: J. Ferrante, J.H. Lee, E.P. McCarthy, K.J. Fisher, E.C. Gonzalez, R.G. Roetzheim.
Analysis and interpretation of the data: J. Ferrante, J.H. Lee, E.P. Mc-Carthy, K.J. Fisher, R. Chen, R.G. Roetzheim.
Drafting of the article: J. Ferrante, K.J. Fisher, E.C. Gonzalez, K. Love-Jackson, R.G. Roetzheim.
Critical revision of the article for important intellectual content: J. Ferrante, E.P. McCarthy, K.J. Fisher, E.C. Gonzalez, R.G. Roetzheim.
Final approval of the article: J. Ferrante, J.H. Lee, E.P. McCarthy, K.J. Fisher, R. Chen, E.C. Gonzalez, K. Love-Jackson, R.G. Roetzheim. Provision of study materials or patients: R.G. Roetzheim.
Statistical expertise: J.H. Lee, E.P. McCarthy, K.J. Fisher, R. Chen. Obtaining of funding: J.H. Lee, R.G. Roetzheim.
Administrative, technical, or logistic support: J. Ferrante, E.P. McCarthy, K. Love-Jackson, R.G. Roetzheim.
Collection and assembly of data: J.H. Lee, E.P. McCarthy, K.J. Fisher, R. Chen, R.G. Roetzheim.
From Rutgers–Robert Wood Johnson Medical School and Cancer Institute of New Jersey, New Brunswick, New Jersey; Moffitt Cancer Center and University of South Florida, Tampa, Florida; and Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts.
An earlier version of this paper was presented at the Cancer and Primary Care Research International Network 5th International Annual Meeting, Cleveland, Ohio, 6 June 2012.
Dr. Roetzheim has full access to all study data and takes responsibility for their integrity and the accuracy of the data analysis and interpretation.
Disclaimer: The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended or inferred.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M13-0347.
Reproducible Research Statement: Study protocol, statistical code, and data set: Not available.
Contributor Information
Dr. Jeanne M. Ferrante, Department of Family Medicine and Community Health, Rutgers–Robert Wood Johnson Medical School, 1 World's Fair Drive, Suite 1500, Somerset, NJ 08873..
Dr. Ji-Hyun Lee, Moffitt Cancer Center, Biostatistics and Bioinformatics Department, 12902 Magnolia Drive, Tampa, FL 33612..
Dr. Ellen P. McCarthy, Beth Israel Deaconess Medical Center, 1309 Beacon Street, Brookline, MA 02446..
Ms. Kate J. Fisher, Moffitt Cancer Center, Biostatistics and Bioinformatics Department, 12902 Magnolia Drive, Tampa, FL 33612..
Dr. Ren Chen, University of South Florida, 3515 East Fletcher Avenue, MDT 1200, Tampa, FL 33612..
Dr. Eduardo C. Gonzalez, University of South Florida, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612..
Ms. Kymia Love-Jackson, University of South Florida, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612..
Dr. Richard G. Roetzheim, University of South Florida, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612..
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [PMID: 23335087]. [DOI] [PubMed] [Google Scholar]
- 2.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [PMID: 18445825]. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [PMID: 18838716]. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Trends Progress Report—2009/2010 Update. National Cancer Institute, National Institutes of Health, Department of Health and Human Services; Bethesda, MD: 2010. [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [PMID: 22237781]. [DOI] [PubMed] [Google Scholar]
- 6.Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30:313–9. doi: 10.1016/j.amepre.2005.11.006. [PMID: 16530618]. [DOI] [PubMed] [Google Scholar]
- 7.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41:23–9. doi: 10.1016/j.ypmed.2004.11.004. [PMID: 15916989]. [DOI] [PubMed] [Google Scholar]
- 8.Hao Y, Jemal A, Zhang X, Ward EM. Trends in colorectal cancer incidence rates by age, race/ethnicity, and indices of access to medical care, 1995–2004 (United States). Cancer Causes Control. 2009;20:1855–63. doi: 10.1007/s10552-009-9379-y. [PMID: 19543799]. [DOI] [PubMed] [Google Scholar]
- 9.Roetzheim RG, Gonzalez EC, Ramirez A, Campbell R, van Durme DJ. Primary care physician supply and colorectal cancer. J Fam Pract. 2001;50:1027–31. [PMID: 11742602]. [PubMed] [Google Scholar]
- 10.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Van Durme DJ, Ayanian JZ, et al. The effects of physician supply on the early detection of colorectal cancer. J Fam Pract. 1999;48:850–8. [PMID: 10907621]. [PubMed] [Google Scholar]
- 11.Ferrante JM, McCarthy EP, Gonzalez EC, Lee JH, Chen R, Love-Jackson K, et al. Primary care utilization and colorectal cancer outcomes among Medicare beneficiaries. Arch Intern Med. 2011;171:1747–57. doi: 10.1001/archinternmed.2011.470. [PMID: 22025432]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colwill JM, Cultice JM, Kruse RL. Will generalist physician supply meet demands of an increasing and aging population? Health Aff (Millwood) 2008;27:w232–41. doi: 10.1377/hlthaff.27.3.w232. [PMID: 18445642]. [DOI] [PubMed] [Google Scholar]
- 13.Okie S. The evolving primary care physician. N Engl J Med. 2012;366:1849–53. doi: 10.1056/NEJMp1201526. [PMID: 22591290]. [DOI] [PubMed] [Google Scholar]
- 14.Starfield B, Chang HY, Lemke KW, Weiner JP. Ambulatory specialist use by nonhospitalized patients in us health plans: correlates and consequences. J Ambul Care Manage. 2009;32:216–25. doi: 10.1097/JAC.0b013e3181ac9ca2. [PMID: 19542811]. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services and Office of Disease Prevention and Health Promotion . Healthy People 2020. US Dept of Health and Human Services; Washington, DC: 2010. Accessed at www.healthypeople.gov/2020/TopicsObjectives2020/pdfs/HP2020_brochure_with_LHI_508.pdf on 31 July 2013. [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV–3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [PMID: 12187163]. [DOI] [PubMed] [Google Scholar]
- 17.Cormen TH, Leiserson CE, Rivest RL, Stein C. Introduction to Algorithms. 3rd ed. MIT Pr; Cambridge, MA: 2009. Greedy Algorithms. [Google Scholar]
- 18.Edmonds J. Matroids and the greedy algorithm. Mathematical Programming. 1971;1:127–36. [Google Scholar]
- 19.Beebe M, Green G, Pavloski D, et al. American Medical Association . Current Procedural Terminology: CPT 2005. American Med Assoc; Chicago, IL: 2004. [Google Scholar]
- 20.Baldwin LM, Adamache W, Klabunde CN, Kenward K, Dahlman C, L Warren J. Linking physician characteristics and medicare claims data: issues in data availability, quality, and measurement. Med Care. 2002;40:IV–82-95. doi: 10.1097/00005650-200208001-00012. [PMID: 12187173]. [DOI] [PubMed] [Google Scholar]
- 21.Valderas JM, Starfield B, Forrest CB, Sibbald B, Roland M. Ambulatory care provided by office-based specialists in the United States. Ann Fam Med. 2009;7:104–11. doi: 10.1370/afm.949. [PMID: 19273864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gornick ME, Eggers PW, Riley GF. Associations of race, education, and patterns of preventive service use with stage of cancer at time of diagnosis. Health Serv Res. 2004;39:1403–27. doi: 10.1111/j.1475-6773.2004.00296.x. [PMID: 15333115] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirschner CG, Burkett RC, Coy JA, Edwards NK, Kotowicz GM, Leoni G, et al. American Med Assoc. Physicians’ Current Procedural Terminology: CPT ‘94. American Med Assoc; Chicago, IL: 1994. [Google Scholar]
- 24.HCPCS general information Centers for Medicare & Medicaid Services Web site. Accessed at www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/index.html on 31 July 2013.
- 25.McCarthy EP, Burns RB, Coughlin SS, Freund KM, Rice J, Marwill SL, et al. Mammography use helps to explain differences in breast cancer stage at diagnosis between older black and white women. Ann Intern Med. 1998;128:729–36. doi: 10.7326/0003-4819-128-9-199805010-00005. [PMID: 9556466]. [DOI] [PubMed] [Google Scholar]
- 26.Schonberg MA, Marcantonio ER, Ngo L, Li D, Silliman RA, McCarthy EP. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol. 2011;29:1570–7. doi: 10.1200/JCO.2010.33.0472. [PMID: 21402602]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [PMID: 3558716]. [DOI] [PubMed] [Google Scholar]
- 28.Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–54. doi: 10.1093/aje/kwm070. [PMID: 17504779]. [DOI] [PubMed] [Google Scholar]
- 29.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–63. [PMID: 9750244]. [PubMed] [Google Scholar]
- 30.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296:2815–22. doi: 10.1001/jama.296.23.2815. [PMID: 17179458]. [DOI] [PubMed] [Google Scholar]
- 31.Lang K, Korn JR, Lee DW, Lines LM, Earle CC, Menzin J. Factors associated with improved survival among older colorectal cancer patients in the US: a population-based analysis. BMC Cancer. 2009;9:227. doi: 10.1186/1471-2407-9-227. [PMID: 19594933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez EC, Roetzheim RG, Ferrante JM, Campbell R. Predictors of proximal vs. distal colorectal cancers. Dis Colon Rectum. 2001;44:251–8. doi: 10.1007/BF02234301. [PMID: 11227943]. [DOI] [PubMed] [Google Scholar]
- 33.Hughes C. What you need to know about the Medicare preventive services expansion. Fam Pract Manag. 2011;18:22–5. [PMID: 21302882]. [PubMed] [Google Scholar]
- 34.Iglehart JK. Entry point. Health Aff (Millwood) 2010;29:758–9. doi: 10.1377/hlthaff.2010.0411. [PMID: 20439857]. [DOI] [PubMed] [Google Scholar]
- 35.Neugut AI, Lebwohl B. Colonoscopy vs sigmoidoscopy screening: getting it right. JAMA. 2010;304:461–2. doi: 10.1001/jama.2010.1001. [PMID: 20664047]. [DOI] [PubMed] [Google Scholar]
- 36.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. PLCO Project Team. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [PMID: 22612596]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrante JM, Balasubramanian BA, Hudson SV, Crabtree BF. Principles of the patient-centered medical home and preventive services delivery. Ann Fam Med. 2010;8:108–16. doi: 10.1370/afm.1080. [PMID: 20212297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starfield B. Reinventing primary care: lessons from Canada for the United States. Health Aff (Millwood) 2010;29:1030–6. doi: 10.1377/hlthaff.2010.0002. [PMID: 20439902]. [DOI] [PubMed] [Google Scholar]
- 39.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88:653–63. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [PMID: 10649261]. [DOI] [PubMed] [Google Scholar]
- 40.Friedberg MW, Hussey PS, Schneider EC. Primary care: a critical review of the evidence on quality and costs of health care. Health Aff (Millwood) 2010;29:766–72. doi: 10.1377/hlthaff.2010.0025. [PMID: 20439859] [DOI] [PubMed] [Google Scholar]