Fig. 1. Experimental geometry of the single-molecule eIF4A helicase assay (not to scale).
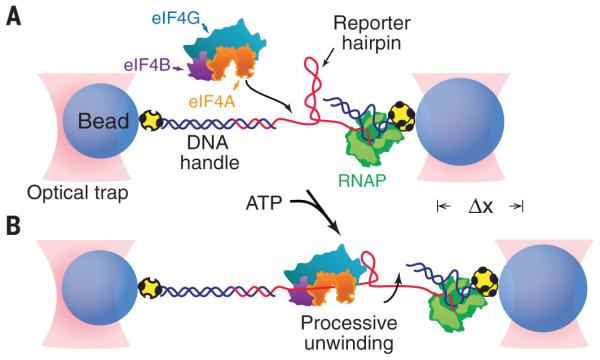
(A) A single 72-bp RNA reporter hairpin (red) is tethered between two microscopic avidincoated beads (blue) held in optical traps (pink) by a DNA handle and a biotinylated RNA polymerase (green) transcriptionally stalled at a biotin-avidin roadblock (yellow). The tether contains a short single-stranded RNA flanking sequence adjacent to the 5′ side of the hairpin for loading eIF4A helicase, shown here complexed with eIF4B and eIF4G. (B) As eIF4A, alone or bound to combinations of eIF4B, eIF4H, and eIF4G, translocates along the RNA, its helicase activity unwinds the hairpin, leading to an increase in distance (Dx) between the trapped beads. In all figures, “eIF4G” corresponds to the truncation mutant eIF4G682-1105.
