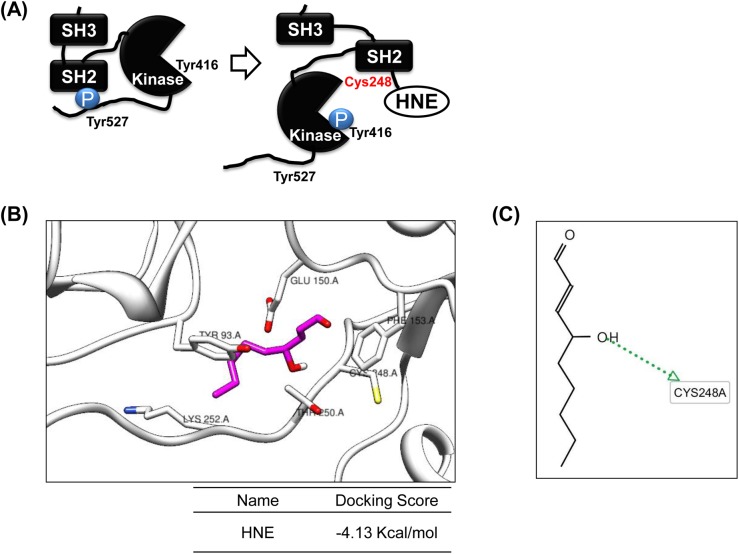
Fig 2. Simulation and conformational modeling of Src modified by 4-HNE.
(A) Conformational modeling of Src modified by 4-HNE at Cys248 in its SH2 domain. (B) Docking simulation for the interaction between Src and 4-HNE using AutoDock 4.2 [42]. The SH2 domain in the crystal structure of human active c-Src constitutes a 4-HNE binding pocket. The binding energy of the 4-HNE/Src interaction was -4.13 kcal/mol. (C) hydrogen bonding between Src and 4-HNE as determined using the Ligand Scout program based on docking simulation results. It was predicted that certain residues of Src, notably Cys248, were mainly responsible for hydrogen bonding between 4-HNE and Src.