SUMMARY
ADAMTS13, encoded on chromosome 9q34, is a member of the ADAMTS (a disintegrin and metalloprotease with thrombospondin type 1 motif) metalloprotease family, containing the common domain structure of (from the amino terminus) signal peptide, propeptide, reprolysin type metalloprotease, thrombospondin type 1 motif, cysteine-rich region, and spacer domain. ADAMTS13 cleaves von Willebrand factor (VWF) in a shear stress dependent manner. Deficiency of the enzyme causes the platelet aggregation of thrombotic thrombocytopenic purpura (TTP). Inhibitory antibodies of ADAMTS13 are detected in patients with acquired TTP, while homozygous or double heterozygous mutations of the ADAMTS13 gene cause the hereditary form of the disease 1. Targeting of the ADAMTS13 gene by recombinant technology has reproduced the phenotype of human TTP in ADAMTS13-null mice 2.
Despite these advances, intense controversy and confusion persist regarding the role of ADAMTS13 assays in the diagnosis of TTP. This brief review highlights some of the contentious issues and proposes steps to improve the diagnostic value of ADAMTS13 assays.
1. ISSUES
(1) Is severe ADAMTS13 deficiency specific for TTP?
The specificity of severe ADAMTS13 deficiency for TTP cannot be resolved by comparing various clinical series of TTP and hemolytic uremic syndrome (HUS), because until recently there was no molecular or pathophysiological basis for distinguishing these two entities. Instead, the specificity is supported by the findings of many studies showing that severe ADAMTS13 deficiency is not found in normal subjects, randomly selected hospitalized patients, patients with E. coli O157:H7 associated or other specific types of thrombotic microangiopathy, and patients with unrelated disorders. Presence of severe ADAMTS13 deficiency in occasional patients without “TTP” merely reflects imprecision of clinical diagnosis or the ADAMTS13 assays. Overall, the available data demonstrates that severe ADAMTS13 deficiency is specific for TTP.
(2) If severe ADAMTS13 deficiency defines TTP, why is it not present in all “TTP” patients?
Two factors contribute to this discrepancy: how TTP is defined and the reliability of ADAMTS13 assays, which will be discussed in a later section.
It is generally agreed that an adolescent or adult presenting with acute thrombocytopenia, microangiopathic hemolysis, mental changes or focal neurological deficits and hematuria with no or minimal renal failure has TTP if there are no other plausible causes. The diagnosis becomes less certain if the patient has co-existing conditions such as autoimmune connective tissue disorders or develops overt renal failure, which is more likely to develop in patients with the hemolytic uremic syndrome.
For studies of diseases with unknown pathogenesis or molecular defects, it is necessary to establish a set of strict criteria to exclude cases whose diagnosis is less certain. This approach to optimize the uniformity of study subjects is not novel; it has been widely used in studies of polycythemia vera and other disorders. The trade-off is that less typical cases will be excluded.
In an extensive review in 1982, Bukowski proposed that for investigational purposes patients with plausible causes, positive anti-nuclear factors or significant renal failure should be excluded from the study of TTP 3. Similarly, we find that after excluding patients with plausible causes or peak Cr > 3.0 mg/dL, the remaining patients are uniformly associated with severe ADAMTS13 deficiency (Table 1) 4,5.
Table 1.
ADAMTS13 deficiency in clinical series of “thrombotic thrombocytopenic purpura”
| Authors | Exclusion of renal failure | Exclusion of secondary cases | Severe deficiency |
|---|---|---|---|
| Tsai, et al 4 | Yesa | Yesc | 100% |
| Zhou, et al 5 | Yesa | Yesc | 100% |
| Furlan, et al 6 | Yesb | No | 83% |
| Veyradier, et al 7 | Yesb | Yesd | 89% |
| Hovinga, et al 8 | Yesb | Yesd | 57% |
| Matsumoto, et al 9 | Yesb | Yesd | 52% |
| Coppo, et al 10 | No | Yesd | 67% |
| Terrel, et al 11 | No | Yesd | 34% |
| Peyvandi, et al 12 | No | No | 48% |
| Bohm, et al 13 | Not stated | No | 91% |
| Rick, et al 14 | Not stated | No | 78% |
| Kokame, et al 15 | Not stated | Not stated | 80% |
Peak Cr > 3.0 mg/dL.
Diagnosis was provided by participating centers using undisclosed criteria.
Based on assessment of the entire courses.
Based on initial clinical assessment or not specified.
Table 1 also shows that severe ADAMTS13 deficiency are found in 34% – 91% of the cases in 10 other series, each with at least 20 cases of “TTP” 6–15. Notably, some series did not exclude patients with either renal failure or plausible causes, while others used diagnoses provided by the referring centers. Thus these series very likely included patients that had other types of thrombotic microangiopathy.
(3) Why are some patients with severe ADAMTS13 deficiency asymptomatic?
Absence of symptoms does not contradict with the diagnosis of TTP. It is common knowledge that all diseases are variable in their presentation due to differences in the genetic makeup and the environmental exposure of the affected individuals. The same holds true for diseases in which a single gene or protein plays the predominant role in the development of the disease phenotype.
It is now clear that the florid manifestations commonly associated with TTP are seen in patients presenting with advanced phase of the disease. Increasingly recognized are asymptomatic patients and patients presenting with isolated thrombocytopenia or strokes. Obviously, it is critical to identify these atypical TTP cases.
(5) How is ADAMTS13 activity measured?
Various assays have been developed to measure the activity of ADAMTS13 in plasma samples. These assays differ in substrates, digestion conditions, need of protease activation, and methods of detecting the cleavage (Table 2). For reliable results, operator experience is also critical.
Table 2.
Characteristic features of ADAMTS13 assays
| Substrate | Method of detection | Target of detection | Comments | |
|---|---|---|---|---|
| VWF multimers | SDS PAGE and immunoblotting 5 | Proteolytic fragments | Validated in a linkage study 24 Technically demanding |
|
| Multimer analysis and immunoblotting | VWF multimers | Technically demanding | ||
| ELISA-Collagen binding 17 | VWF multimers | Performance is variable 23 | ||
| Ristocetin cofactor activity 14 | VWF multimers | Good performance in a blind study 23 | ||
| IRMA18 | VWF multimers | Limited experience | ||
| ELISA-Ab774619 | VWF multimers | Limited experience | ||
| VWF peptides | GST-1596VWF1668-His | ELISA6 | VWF peptides | Used in our clinical lab |
| Hrp-1591VWF1668-biotin | HRP activity after adsorption with avidin 21 | Proteolytic fragments | Limited experience | |
| His-A2-Tag100 | ELISA20 | VWF peptides | Limited experience | |
| FREST-VWF73 | Fluorescence 16 | Proteolytic fragments | Quick turnaround Cost may be prohibiting |
|
Original ADAMTS13 assays use plasma-derived VWF multimers as the substrate and require a step of conformational unfolding with either urea or guanidine HCl 4,6,13,16,–18. Recombinant Type 2A VWF multimers and several recombinant VWF fragments have been produced that are constitutively susceptible to cleavage by ADAMTS13 5,15,19,20.
The substrate digestion step may require overnight incubation if it is dialyzed against urea. After the digestion step, some assays measure the residual VWF multimers or peptides 5,6,13,16,–19, while others measure the digestion products 4,15,20. Measurement of residual VWF multimers may yield falsely lower activity results for plasma samples containing high VWF concentrations, unless the VWF is depleted before the assay 18. This interference may account for the inverse correlation between VWF and ADAMTS13 levels that some investigators have observed 15,18,21.
(6) How should the performance of an ADAMTS13 assay be assessed?
Because the primary use of an ADAMTS13 assay is to identify patients with TTP due to severe ADAMTS13 deficiency, evaluation of an assay performance should focus on the range of 0% – 20%. The international collaborative study demonstrates that only one of the 11 assays evaluated in the study produced acceptable and reproducible results in this range 22. Separately, only one assay has been validated in a genetic linkage study of congenital TTP 23. Thus, assay variability may have contributed to the confusion and uncertainty in the interpretation of ADAMTS13 assay results. The range of 20% –100% is not relevant for diagnosis of TTP but is useful for follow-up studies of TTP patients and for comparison of samples from patients without TTP.
The international study was limited to two plasma samples mixed at various ratios and might not reflect the performance of the assays in practice.
(7) Are there non-inhibitory ADAMTS13 antibodies?
Analysis of mixtures of patient and normal plasma samples produces positive inhibitory results in 50% – 90% of the cases with severe ADAMTS13 deficiency 24. Since it does not detect low levels of inhibitors, a negative mixing study does not preclude the presence of inhibitors, and should not be regarded as an indicator that the antibodies are non-inhibitory. Most of the patients with negative mixing study results exhibit inhibitory activity when their IgG molecules are isolated and tested at high concentrations.
ADAMTS13 binding IgG is detectable in 97% – 100% of samples with severe ADAMTS13 deficiency and obtained before blood or plasma transfusion 24–26. However, the specificity of ADAMTS13-binding IgG assays may be ≤90%. Inclusion of a competitive inhibition step with unbound ADAMTS13 improves the specificity of the assay 24.
Measurement of ADAMTS13 antigen levels produces variable results in TTP. The reliability of ADAMTS13 antigen measurement requires further investigation.
2. RECOMMENDATIONS
(1) Samples
In some of the plasma samples obtained from patients with a variety of conditions, the activity of ADAMTS13 is unstable (Figure 1). To minimize this in-vitro loss, socium citrate anticoagulated plasma samples should be free of clotting and tested without delay. Serum samples frequently yield falsely low levels.
Figure 1. Decline of ADAMTS13 activity in two plasma samples.
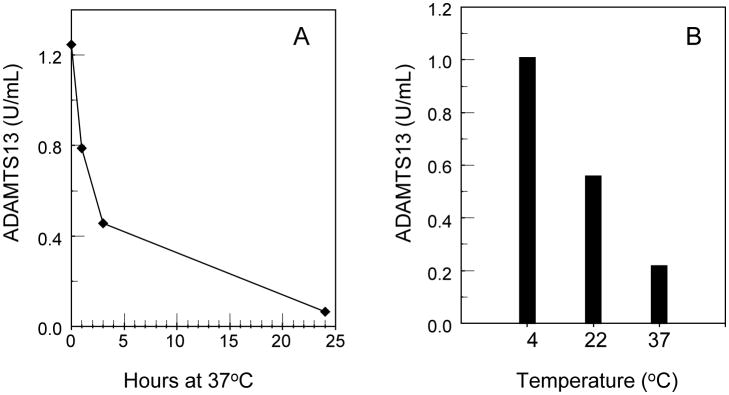
A: Time-dependent decline of ADAMTS13 activity in one sample at 37°C.
B: Temperature dependent decline of ADAMTS13 activity in another plasma sample after incubation for 24 hours.
(2) Assay conditions
Ideally the plasma samples should be incubated for no more than one hour at 37 °C. Assays requiring longer incubations may yield lower values because ADAMTS13 may decay. Overnight incubation at 37°C should be avoided if possible. Low pH may promote inactivation of the protease. For assays that do not directly visualize the size of the digestion fragments, addition of protease inhibitors may minimize erroneous results due to extraneous enzymes.
(3) Quality control
As the international collaborative study clearly demonstrates, quality control is critically important 22. Defining the normal range and coefficient of variance is essential but may not be sufficient to establish the reliability of an assay. Each laboratory should vigorously examine the performance of its assay on a large variety of samples. Each run of assay should include a reference curve, a separate normal control sample, and an abnormal sample with ADAMTS13 activity in the range of 0% – 20%. Questionable results should be verified with assays based on different designs.
(4) When is ADAMTS13 measurement indicated?
Analysis of ADAMTS13 is indicated whenever TTP is suspected, but is particularly instrumental for patients presenting with atypical features. An ADAMTS13 assay may also provide critical information during the course of therapy and clinical remission.
In a patient with TTP, the platelet count may fail to normalize after plasma exchange for other reasons such as autoimmune thrombocytopenic purpura (particularly in patients with HIV infection) or heparin-induced autoimmune response. In such cases, a detectable level of ADAMTS13 greater than 10% of control helps exclude TTP as the cause of thrombocytopenia.
Patients have widely different levels of ADAMTS13 during remission. A patient with a level ≤ 10% – 15% of normal control is predisposed to relapse when stressed with fever, infection, surgery or pregnancy. Further studies are needed to determine whether serial ADAMTS13 analysis will predict the risk of unprovoked relapse.
(5) Interpretation of assay results
Because the propensity to platelet thrombosis in association with severe ADAMTS13 deficiency is likely to be affected by various genetic or environmental factors, the ADAMTS13 threshold levels for causing manifestation of TTP differ among individual patients and may be lower than the level used for exclusion of TTP. Each laboratory should establish its own threshold values. Sequential ADAMTS13 analysis in individual cases may be more informative than a single measurement.
A very low ADAMTS13 value (< 15% – 20%) without evidence of compromised VWF proteolysis raises the possibility that it does not reflect the correct ADAMTS13 level of the patient.
(6) Literature review
Differences in the assays and in the selection of study subjects are critical factors for proper interpretation of the clinical series. Indiscriminate comparison of clinical studies fuels confusions. For individual cases, the experience of the laboratory is more relevant than a cursory review of the literature.
Acknowledgments
Supported in part by grants R01 HL62136 and R01 HL72876 from the National Heart, Lung and Blood.
References
- 1.Tsai HM. Current concepts in thrombotic thrombocytopenic purpura. Ann Rev Med. 2006;57:419–436. doi: 10.1146/annurev.med.57.061804.084505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115:2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukowski RM. Thrombotic thrombocytopenic purpura. A review. Prog Haemost Thromb. 1982;6:287–337. [PubMed] [Google Scholar]
- 4.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Tsai HM. An enzyme immunoassay of ADAMTS13 distinguishes patients with thrombotic thrombocytopenic purpura from normal individuals and carriers of ADAMTS13 mutations. Thromb Haemost. 2004;91:806–811. doi: 10.1160/TH03-11-0675. [DOI] [PubMed] [Google Scholar]
- 6.Furlan M, Robles R, Galbusera M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 7.Veyradier A, Obert B, Houllier A, et al. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98:1765–1772. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 8.Hovinga JA, Studt JD, Alberio L, et al. von Willebrand factor-cleaving protease (ADAMTS-13) activity determination in the diagnosis of thrombotic microangiopathies: the Swiss experience. Semin Hematol. 2004;41:75–82. doi: 10.1053/j.seminhematol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, Yagi H, Ishizashi H, et al. The Japanese experience with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Semin Hematol. 2004;41:68–74. doi: 10.1053/j.seminhematol.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Coppo P, Bengoufa D, Veyradier A, et al. Severe ADAMTS13 deficiency in adult idiopathic thrombotic microangiopathies defines a subset of patients characterized by various autoimmune manifestations, lower platelet count, and mild renal involvement. Medicine (Baltimore) 2004;83:233–244. doi: 10.1097/01.md.0000133622.03370.07. [DOI] [PubMed] [Google Scholar]
- 11.Terrell DR, Williams LA, Vesely SK, et al. The incidence of thrombotic thrombocytopenic purpurahemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J Thromb Haemost. 2005;3:1432–1436. doi: 10.1111/j.1538-7836.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- 12.Peyvandi F, Ferrari S, Lavoretano S, et al. von Willebrand factor cleaving protease (ADAMTS-13) and ADAMTS-13 neutralizing autoantibodies in 100 patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2004;127:433–439. doi: 10.1111/j.1365-2141.2004.05217.x. [DOI] [PubMed] [Google Scholar]
- 13.Bohm M, Vigh T, Scharrer I. Evaluation and clinical application of a new method for measuring activity of von Willebrand factor-cleaving metalloprotease (ADAMTS13) Ann Hematol. 2002;81:430–435. doi: 10.1007/s00277-002-0502-3. [DOI] [PubMed] [Google Scholar]
- 14.Rick ME, Moll S, Taylor MA, et al. Clinical use of a rapid collagen binding assay for von Willebrand factor cleaving protease in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2002;88:598–604. [PubMed] [Google Scholar]
- 15.Kokame K, Nobe Y, Kokubo Y, et al. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 16.Gerritsen HE, Turecek PL, Schwarz HP, et al. Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF: a tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP) Thromb Haemost. 1999;82:1386–1389. [PubMed] [Google Scholar]
- 17.Obert B, Tout H, Veyradier A, et al. Estimation of the von Willebrand factor-cleaving protease in plasma using monoclonal antibodies to vWF. Thromb Haemost. 1999;82:1382–1385. [PubMed] [Google Scholar]
- 18.He S, Cao H, Magnusson CG, et al. Are increased levels of von Willebrand factor in chronic coronary heart disease caused by decrease in von Willebrand factor cleaving protease activity? A study by an immunoassay with antibody against intact bond 842Tyr-843Met of the von Willebrand factor protein. Thromb Res. 2001;103:241–248. doi: 10.1016/s0049-3848(01)00320-6. [DOI] [PubMed] [Google Scholar]
- 19.Whitelock JL, Nolasco L, Bernardo A, et al. ADAMTS-13 activity in plasma is rapidly measured by a new ELISA method that uses recombinant VWF-A2 domain as substrate. J Thromb Haemost. 2004;2:485–491. doi: 10.1111/j.1538-7836.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu JJ, Fujikawa K, Lian EC, et al. A rapid enzyme-linked assay for ADAMTS-13. J Thromb Haemost. 2006;4:129–136. doi: 10.1111/j.1538-7836.2005.01677.x. [DOI] [PubMed] [Google Scholar]
- 21.Mannucci PM, Capoferri C, Canciani MT. Plasma levels of von Willebrand factor regulate ADAMTS-13, its major cleaving protease. Br J Haematol. 2004;126:213–218. doi: 10.1111/j.1365-2141.2004.05009.x. [DOI] [PubMed] [Google Scholar]
- 22.Tripodi A, Chantarangkul V, Bohm M, et al. Measurement of von Willebrand factor cleaving protease (ADAMTS-13): results of an international collaborative study involving 11 methods testing the same set of coded plasmas. J Thromb Haemost. 2004;2:1601–1609. doi: 10.1111/j.1538-7836.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 23.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 24.Tsai HM, Raoufi M, Zhou W, et al. ADAMTS13-binding IgG are present in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2006;95:886–892. [PMC free article] [PubMed] [Google Scholar]
- 25.Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262–1267. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 26.Shelat SG, Smith P, Ai J, Zheng XL. Inhibitory autoantibodies against ADAMTS-13 in patients with thrombotic thrombocytopenic purpura bind ADAMTS-13 protease and may accelerate its clearance in vivo. J Thromb Haemost. 2006;4:1707–1717. doi: 10.1111/j.1538-7836.2006.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


