Abstract
Background
Many epidemiological studies analyze the relationship between hyperuricemia and cardiovascular outcomes. This observational prospective study investigates the association of serum uric acid (SUA) levels with adverse cardiovascular events and deaths in an elderly population affected by advanced atherosclerosis.
Methods
Two hundred and seventy six elderly patients affected by advanced atherosclerosis (217 males and 59 females; aged 71.2 ± 7.8 years) were included. All patients were assessed for history of cardiovascular disease, cancer, obesity and traditional risk factors. Patients were followed for approximately 31 ± 11 months. Major events were recorded during follow-up, defined as myocardial infarction, cerebral ischemia, myocardial and/or peripheral revascularization and death.
Results
Mean SUA level was 5.47 ± 1.43 mg/dL; then we further divided the population in two groups, according to the median value (5.36 mg/dL). During a median follow up of 31 months (5 to 49 months), 66 cardiovascular events, 9 fatal cardiovascular events and 14 cancer-related deaths have occurred. The patients with increased SUA level presented a higher significant incidence of total cardiovascular events (HR: 1.867, P = 0.014, 95% CI: 1.134–3.074). The same patients showed a significant increased risk of cancer-related death (HR: 4.335, P = 0.025, 95% CI: 1.204–15.606).
Conclusions
Increased SUA levels are independently and significantly associated with risk of cardiovascular events and cancer related death in a population of mainly elderly patients affected by peripheral vasculopathy.
Keywords: Cardiovascular events, Peripheral artery disease, Uric acid
1. Introduction
Cardiovascular and cancer disease are the main cause of morbidity and mortality in the western and developing world.[1] At same time, as the mean lifespan of human population is growing over time, the increase incidence of disease in the elderly is associated with multiple comorbidities requiring a more incisive and global approach, in term of drug treatments and lifestyle changing.[2]
For several years, traditional risk factors have been taking into account in worldwide guidelines driving medical approach to cardiovascular disease, leading to an important reduction of mortality of patients;[3] despite these huge efforts and the encouraging achieved results, we still observe residual cardiovascular risk we need to address.[4]
Recently, there has been a growing interest in the study of hyperuricemia and its association with various diseases, beyond the gout, such as hypertension, atherosclerosis, cardiovascular disease and chronic kidney diseases.[5],[6] Serum acid uric (SUA) is the product of oxidation of xanthine and hypoxanthine by xanthine oxidoreductase, pharmacologically inhibited by commonly used drugs such as allopurinol and febuxostat in the treatment of hyperuricemia related to gout and nephrolithiasis.[7] SUA takes important part in multiple metabolic, homeostatic and hemodynamic abnormalities such as insulin resistance, abdominal obesity, dyslipidemia and several studies indicate SUA as a key ingredient in the setting of metabolic syndrome.[8] Several in vitro and in vivo studies showed SUA implication in oxidative stress, inflammatory response and its key role in endothelial function and vascular remodeling.[9],[10] Recent epidemiological and experimental analysis suggest an important association between hyperuricemia and cancer disease.[11],[12]
At this time, there is wide debate on treatment of uncomplicated asymptomatic hyperuricemia.[13],[14] Whether uric acid levels is a marker for residual cardiovascular risk or cancer disease, we need to take it into account to build new risk chart to better predict outcome for each population of interest. The most part of previous studies on hyperuricemia concerns on patient at not very high risk for cardiovascular events.
This paper describes a prospective epidemiological study, concerning a very high risk population, mainly living in Puglia, in the South Italy, homogenous for clinical condition and traditional cardiovascular risk factor, affected by advanced peripheral artery disease, followed in outpatient clinic. The main aim of our study is to explore an eventual role of serum uric acid as a possible risk factor for major cardiovascular event and death for any cause.
2. Methods
Two hundred and seventy six elderly patients affected by advanced atherosclerosis (217 males and 59 females; aged 71.2 ± 7.8 years), consecutively attending our clinic from November 2011 to November 2013, were studied. The inclusion criteria was identified by advanced atherosclerosis, defined as significant carotid stenosis (> 50% on the basis of velocimetry measurement by Doppler analysis) and/or lower limb ischemia II or III stadium Leriche-Fontaine (evaluated by treadmill test at 12% inclination and 3.5 km/h speed). The exclusion criteria were represented by mild carotid atheroma (intimal thickening or plaques determinants stenosis less than 50%), asymptomatic arterial disease (Leriche-Fontaine stage I), gangrene of the lower limbs (Leriche-Fontaine stage IV) and cancer with an expectation life less than 6 months.
All patients were assessed for history of cardiovascular disease, cancer, cigarette smoking, obesity (body mass index and waist-hip ratio), arterial hypertension (≥ 130/85 mmHg) or antihypertensive treatment, dyslipidemia [high density lipoprotein (HDL) cholesterol < 40 mg/dL in male and < 50 mg/dL in female and/or triglycerides ≥ 150 mg/dL] or antidyslipidemic treatment, type 2 diabetes mellitus, according to criteria of World Health Organization and ATP III.
Presence of previous cardiovascular disease was defined as myocardial infarction, stroke, previous carotid or lower limb revascularization, myocardial surgical and/or endovascular reperfusion procedure (clinical history and antecedent cardiovascular events were assessed by strict analysis of previous hospitalization by hospital discharge letters and defined “present” after appropriate and careful evaluation of clinical documentation).
Patients were assessed for biochemical parameters, such as uric acid, fasting glucose, homeostatic model assessment-insulin resistance (HOMAir), fibrinogen, high sensitivity C reactive protein, urinary albumin, and estimated glomerural filtration rate (eGFR) according to modification of diet in renal disease study.
Patients were subjected to measurement of arterial stiffness, performed by non-invasive method of brachial-ankle pulse wave velocity (PWV) and augmentation index using the AngE System (Sonotechnik, Austria). PWV and augmentation index measurements were obtained in duplicate; the mean of the measurements was used in data analyses. Doppler ultrasound has been used to measure renal resistance index. Echocardiographic examination was performed with MyLab50 Ultrasound System (Esaote). With patients in left lateral decubitus, were measured interventricular septum, left ventricular posterior wall and end-diastolic left ventricular diameter in parasternal long-axis; left ventricular mass was calculated using the Devereux formula. Ventricular mass index was obtained by dividing left ventricular mass to body surface. Electrocardiographic parameters (PR, QRS, QTc intervals, bundle branch block) were measured from the standard 12-lead electrocardiogram.
Patients were followed for approximately 31 ± 11 months. During follow-up, major events, defined as myocardial infarction, cerebral ischemia, myocardial and/or peripheral revascularization and death were recorded. For cardiovascular death, we have included all deaths from heart failure, myocardial infarction, stroke, sudden death, and malignant arrhythmias.
Statistical analysis was conducted using SPSS 13.0 software (Chicago, IL, USA). All continuous data are presented as means ± SD. Quantitative data variables were analyzed by t-test between the two groups, after analysis of normal distribution using Kolmogorov-Smirnov test. Qualitative dichotomous data variables difference between the two groups were analyzed using Pearson's Chi-squared test. The correlation between different variables was assessed using correlation analysis. Multivariate Cox proportional hazards models were applied to assess the association between SUA and cardiovascular events (displayed by Kaplan-Meyer graphic) and deaths. Results of Cox proportional hazards models are presented as hazard ratios (HR) and 95% confidence intervals (CI). To account for potential confounders, we calculated the risk for cardiovascular events by multivariate Cox proportional hazard models adjusted for age, gender, waist circumference, smoking, hypertension, diabetes mellitus, dyslipidemia, renal function and diuretic treatment. All statistical tests were two-sided; values of P < 0.05 were considered statistically significant.
3. Results
The mean age of the 276 study participants was 71.2 ± 7.8 years (92% of population aged more than 60 years). Mean serum uric acid was 5.47 ± 1.43 mg/dL (range 2.46–10.69 mg/dL). Baseline patient characteristics, comorbidities and drug treatments are summarized in Table 1 and 2. Then we further divided the population in two groups, according to the median value (5.36 mg/dL) of SUA level, named low SUA (mean SUA level 4.34 mg/dL, range 2.46–5.35 mg/dL) and high SUA group (mean SUA level 6.61 mg/dL, range 5.37–10.69 mg/dL). The high SUA patients were significantly more obese with an increased waist to hip ratio, higher triglycerides level and more insulin resistant. The deterioration of renal function expressed by decreased eGFR and microalbuminuria was significantly higher in the same group. At the same time, these patients showed reduced ankle brachial index and were more affected by peripheral arterial disease stage II Leriche-Fontaine.
Table 1. Baseline characteristics of study population and the two groups according to SUA levels.
| Total (n = 276) | Group 1 | Group 2 | P value | |
| Age, yrs | 71.2 ± 7.8 | 70.75 ± 8.45 | 71.6 ± 7.1 | NS |
| Men/women | 217/59 | 98/40 | 119/19 | 0.002 |
| BMI, kg/m2 | 28.4 ± 3.9 | 28.0 ± 3.8 | 28.8 ± 3.9 | NS |
| Waist circumference, cm | 101 ± 10.5 | 99 ± 10 | 103 ± 11 | < 0.001 |
| Waist-hip ratio | 0.97 ± 0.07 | 0.95 ± 0.07 | 0.98 ± 0.07 | 0.001 |
| SBP, mmHg | 133 ± 17 | 133 ± 17 | 133 ± 17 | NS |
| DBP, mmHg | 79 ±6 | 80 ± 6 | 79 ± 6 | NS |
| Pulse pressure, mmHg | 54 ± 15 | 54 ± 16 | 54 ± 15 | NS |
| Fasting glucose, mmol/L | 6.56 ± 2.12 | 6.57 ± 1.32 | 6.56 ± 2.01 | NS |
| HOMAir | 4.99 (0.34–125.29) | 4.08 (0.34–62.28) | 5.94 (0.60–125.29) | 0.006 |
| Triglycerides, mg/dL | 118 (40-374) | 108 (51–305) | 128 (40-374) | 0.006 |
| Cholesterol, mg/dL | 164 ± 38 | 162 ± 38 | 166 ± 37 | NS |
| HDL-cholesterol, mg/dL | 48 ± 13 | 50 ± 12 | 47 ± 13 | NS |
| LDL-cholesterol, mg/dL | 92 ± 32 | 91 ± 32 | 94 ± 32 | NS |
| Serum uric acid, mg/dL | 5.47 ± 1.43 | 4.34 ± 0.69 | 6.60 ± 1.03 | < 0.001 |
| Fibrinogen, mg/dL | 349 ± 71 | 348 ± 71 | 353 ± 72 | NS |
| hs-CRP, mg/dL | 0.69 (0.20–33) | 0.77 (0.20–33) | 0.61 (0.20–4.00) | NS |
| Serum creatine, mg/dL | 1.04 ± 0.54 | 1.01 ± 0.32 | 1.17 ± 0.67 | < 0.001 |
| eGFR, mL/min per 1.73 m2 | 80.3 ± 26.1 | 87.7 ± 23.7 | 72.9 ± 26.3 | < 0.001 |
| Microalbuminuria, µg/min | 99.1 (0.5–2250) | 75.8 (2–2250) | 122.4 (0.5–1230) | 0.002 |
| RRI | 0.68 ± 0.07 | 0.7 ± 0.08 | 0.679 ± 0.06 | NS |
| ABI | 0.94 ± 0.22 | 0.97 ± 0.27 | 0.91 ± 0.13 | 0.068 |
| Augmentation index, % | 23.1 ± 7.8 | 22.7 ± 6.9 | 23.6 ± 8.6 | NS |
| PWV, m/s | 14.9 ± 5.9 | 15.3 ± 6.8 | 14.5 ± 4.7 | NS |
| Ventricular mass index, g/m2 | 76.9 ± 20.7 | 76.6 ± 20.5 | 77.2 ± 20.9 | NS |
Data are presented as means ± SD or mean (range) unless other indicated. ABI: ankle brachial index; BMI: Body Mass Index; DBP: diastolic blood pressure; eGFR: glomerular filtration rate; HOMAir: homeostatic model assessment-insulin resistance; hs-CRP: high sensitivity C reactive protein; NS: not significant; PWV: pulse wave velocity; RRI: renal resistance index; SBP: systolic blood pressure; SUA: serum acid uric.
Table 2. Comorbidities and drug treatment.
| Total (n = 276) | Group 1 | Group 2 | P | |
| Comorbidity | ||||
| Hypertension | 255 (92) | 125 (91) | 130 (94) | NS |
| Dyslipidemia | 260 (94) | 131 (95) | 129 (94) | NS |
| Type 2 diabetes | 147 (53) | 72 (52) | 75 (54) | NS |
| Obesity | 144 (55) | 62 (47) | 82 (63) | 0.01 |
| Smoke | 64 (23) | 33 (24) | 31 (23) | NS |
| Leriche/Fontaine | ||||
| I | 146 (53) | 84 (61) | 62 (45) | 0.013 |
| IIA | 78 (28) | 36 (26) | 42 (30) | |
| IIB | 52 (19) | 18 (13) | 34 (25) | |
| Drug therapy | ||||
| ARBs | 108 (39) | 49 (36) | 59 (43) | NS |
| ACE inhibitors | 107 (39) | 47 (34) | 60 (44) | NS |
| Calcium channel blockers | 85 (31) | 42 (30) | 43 (31) | NS |
| β-blockers | 73 (26) | 36 (26) | 37 (27) | NS |
| Diuretics | 119 (43) | 45 (33) | 74 (54) | < 0.001 |
| Anti-platelet | 238 (86) | 119 (86) | 119 (86) | NS |
| Statin | 242 (88) | 123 (89) | 119 (86) | NS |
| Allopurinol and/or febuxostat* | 29 (11) | 17 (12) | 12 (9) | NS |
| Anti-diabetic therapy | ||||
| Diet | 46 (31) | 21 (29) | 25 (34) | 0.048 |
| Oral hypoglycemic | 68 (46) | 40 (56) | 28 (37) | |
| Insulin + oral hypoglycemic | 33 (23) | 11 (15) | 22 (29) |
Data are presented as n (%). *Only one patient was treated by febuxostat. ACE inhibitors: angiotensin converting enzyme inhibitors; ARBs: angiotensin receptor blockers; NS: not significant.
At baseline, the distribution of the traditional cardiovascular risk parameters as hypertension, HDL- and LDL-cholesterol, type 2 diabetes, smoking history, did not differ between the two groups. There was no difference regarding the previous clinical history of myocardial infarction, stroke, cardiac and peripheral revascularization and cancer history (Table 3).
Table 3. Clinical history.
| Total (n = 276) | Group 1 | Group 2 | P | |
| Myocardial infarction | 47 (17) | 28 (20) | 19 (14) | NS |
| Stroke | 35(13) | 22 (16) | 13 (9) | NS |
| Carotid revascularization | 62 (23) | 28 (20) | 34 (25) | NS |
| Lower limb revascularization | 42 (15) | 22 (16) | 20 (15) | NS |
| Myocardial revascularization | 77 (30) | 41 (30) | 36 (26) | NS |
| Cancer | 71 (26) | 32 (23) | 39 (28) | NS |
Data are presented as n (%).NS: not significant.
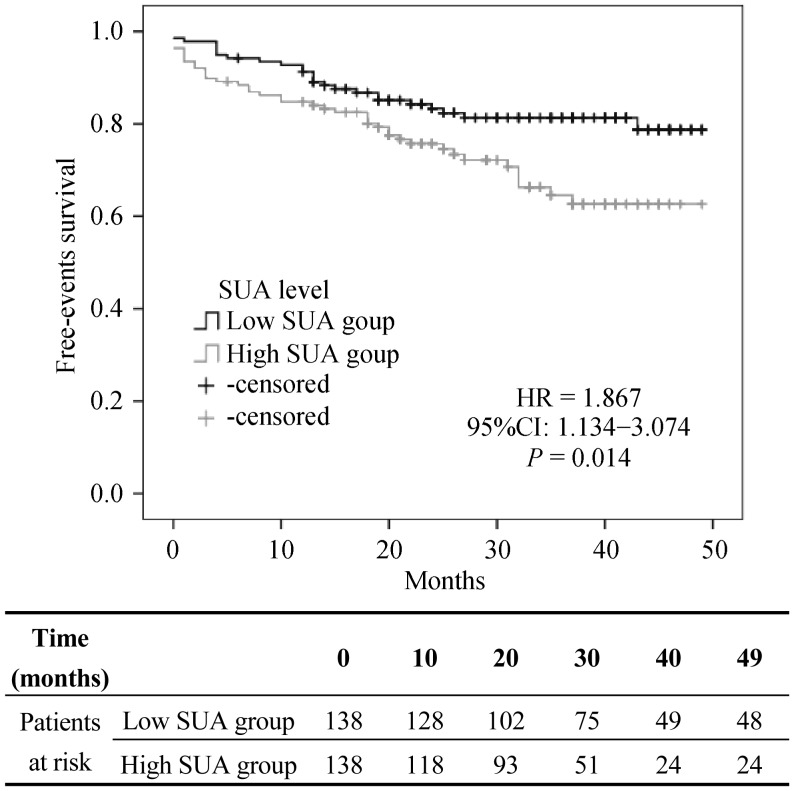
Our findings showed an increased diuretic use in the patients with elevated SUA level, without any other difference in drug treatment. At same time, we noticed a significant difference in eGFR in the patients treated by diuretics (eGFR: 86.27 ± 24.33 mL/min per 1.73 m2 in untreated patients vs. 72.66 ± 26.30 mL/min per 1.73 m2 in treated patients, P < 0.001); therefore, as showed below, we consider diuretic treatment as a confounder factor by multivariate analysis. During a median follow up of 31 months (5 to 49 months), 66 cardiovascular events, 9 fatal cardiovascular events and 14 cancer-related deaths have occurred, as showed in Table 4. The patients with increased SUA level presented a higher significant incidence of total cardiovascular events, defined as cardiac death, cardiac and peripheral revascularization, myocardial infarction and stroke (HR: 1.867, P = 0.014, 95% CI: 1.134–3.074, Figure 1), while there was no difference related to cardiovascular fatal events only. The Cox regression analysis for SUA level as a continue value showed HR: 1.198, P = 0.023, 95% CI: 1.025–1.400 with a risk ratio of 20% (2%–40%) for cardiovascular events for each 1 mg/dL of SUA level.
Table 4. Events at follow up.
| Total (n = 276) | Low SUA Group | High SUA Group | HR (95% CI) | P value | |
| Cardiovascular event during follow-up | 66 (24) | 25 (18) | 41 (30) | 1.867 (1.134–3.074) | 0.014 |
| Death due to cardiovascular event | 9 (3) | 5 (4) | 4 (3) | 0.901 (0.242–3.357) | 0.876 |
| Death due to cancer | 14 (5) | 3 (2) | 11 (8) | 4.335 (1.204–15.606) | 0.025 |
Data are presented as n (%). SUA: serum acid uric.
Figure 1. Cardiovascular events.
SUA: serum acid uric.
This difference persists also after correction for confounding factor (namely age, smoking, gender, diabetes, eGFR, hypertension, dyslipidemia, HOMAir and diuretic treatment) (HR: 1.932, P = 0,025, 95% CI: 1.087–3.433). The same patients showed a significant increased risk of cancer-related death (HR: 4.335, P = 0.025, 95% CI: 1.204–15.606), (Table 4).
4. Discussion
This study analyzes the association between serum uric acid and incidence of cardiovascular events and death for any cause occurred in a short-term of follow up in a cohort of mainly elderly patient (92% of population aged more than 60 years) affected by advanced atherosclerosis in the southern Italy. In our population, none is affected by gout or any symptomatic manifestation of hyperuricemia, and there is no difference in drug treatment between the two groups, considering allopurinol or febuxostat (only one patient was treated by the last one).
SUA levels in our population are under common cut-off level (hyperuricemia defined as SUA ≥ 6 mg/dL), as the mean value is 5.47 ± 1.43 mg/dL, while median value is 5.36 mg/dL, according with line between groups defined; this means, as reported by Desideri, et al.,[15] that our population have normal level of serum uric acid (normal range of value 3.5 to 7.2 mg/dL in men and post-menopausal women in Italian population). Furthermore, as showed in previous scientific literature, higher SUA level are associated with more pronounced expression of key factor of metabolic syndrome. Despite most patients are overweight (mean BMI 28.4 ± 3.9 kg/m2), the High SUA group present a higher abdominal obesity (as indicated by waist circumference), higher level of triglycerides and insulin resistance, expressed by HOMAir. Nevertheless, there is no difference between groups concerning previous cardiovascular and cancer history, traditional risk factors intended as hypertension, diabetes, dyslipidemia, smoke status, neither about pharmacological treatment, with exception of diuretic therapy. Several underlying mechanisms might be responsible for the association between SUA levels and cardiovascular events. Uric acid could contribute to oxidative metabolism, endothelial dysfunction, platelet adhesiveness or aggregation, and vascular smooth muscle cell proliferation in atherosclerosis. The scientific literature show that elevated SUA levels were strongly associated with metabolic syndrome, type 2 diabetes, early stage kidney injury and progressive renal disease, all of which could lead to atherosclerosis progression, acute cardiovascular events and greater mortality.[16]
Our study focuses on a very-high risk elderly population, affected by known peripheral atherosclerosis with high prevalence of previous major cardiovascular events (stroke, myocardial infarct, peripheral and cardiac revascularization) and presenting traditional risk factors already managed by a tailored treatment approach. Most part of previous studies analyze younger population affected by gout and high SUA levels, while another part is represented by large epidemiological studies with medium[17] or long-term follow up,[18],[19] enrolling patient from general population at not very high cardiovascular risk, as self-evident after looking at difference in events incidence. Strazullo and Puig[20] analyzed different prospective studies reviewing the role of uric acid in cardiovascular outcome in different setting; they generally concluded that SUA is a significant independent predictor among subjects at high or very high cardiovascular risk, while its role in low risk population is not established. A large perspective study by Strasak, et al.[21] showed that high SUA levels are independent risk factor for cardiovascular event in patient at high risk, yet this relationship was lacking after adjustment for confounding factors.
Our findings showed an increased diuretic use in the patients with elevated SUA level, as underlined in previous studies.[22] As highlighted in scientific literature, SUA level associated with renal function impairment, in particular in stage K-DOQI (Kidney Disease Outcomes Quality Initiative) 1 and 2 of chronic kidney disease.[23] Our data confirmed microvascular and macrovascular impairment in High SUA group, according with different level of microalbuminuria and eGFR, respectively.
We showed that higher SUA levels associated with death from cancer, despite homologous distribution of cancer disease in the two groups at the enrolling time. In agreement to literature,[24] this could be the expression of an higher cell turnover, specific of aggressive progression of malignant neoplasms; therefore cancer itself could promote hyperuricemia through tumor related cell death rather than being an independent risk factor for the development of cancer. At the same time, SUA level are expression of oxidative stress, chronic inflammation and metabolic syndrome, a pathophysiological milieu that could induce epigenetic modification and cancer growth.
PWV, despite increased according to the expected arterial stiffness in these very high risk patient, did not differ between the two group, and did not provide further stratification for cardiovascular event (data not showed). Preceding researches, concordant with Rotterdam study,[25] demonstrated a clear role of arterial stiffness in stratification of cardiovascular risk in general population, not confirmed elsewhere.[26] Our interpretation suggests that in the setting of advanced atherosclerosis, PWV reaches a plateau level, beyond which it does not more supply risk estimation. According to scientific literature, uric acid could provide an assessment of the functional state of atherosclerotic plaques, as a result of systemic oxidative stress and inflammatory condition, while PWV is a result of the calcium burden of the conductance vessels; the first betting on acute events, the last witnessing a chronic condition.
Our study follows an observational prospective approach, therefore we can only infer association link, more than causal relationship; however this limit is common to many previous researches. Based on our results, acid uric can provide a further estimation of residual cardiovascular risk in patient affected by advanced atherosclerosis. Nevertheless at this moment SUA would not be identified as a therapeutic target to treat by xanthine oxidoreductase inhibitors, as previous clinical studies are not concordant on this statement. However, high SUA level could identify more frail patients, which we have to care more intensively by drug treatment, strict follow up and by right educational and environmental approach.
This is a small, single-center study with a short time follow up, as a matter of intrinsic limit. Nevertheless, the population is homogeneous and well-defined for clinical and therapeutic characteristic. A longer follow up and the enlargement of patient number could provide a better understanding on the role of uric acid in elderly patients affected by advanced atherosclerosis.
In conclusion, on the basis of our finding and previous literature, serum uric acid could be considered as a marker more than a risk factor for cardiovascular event and cancer related-death, expression of the oxidative stress, like a smoking cloud of a dangerous fire. In our population, at very high risk of cardiovascular events uric acid could provide further prognostic stratification. Furthermore, we need additional researches to address the question concerning a valid clinical translation of therapeutic treatments available in this setting, since available data from scientific literature do not show clear clinical benefit.
Acknowledgments
The authors thank Dr. Andrea Fontana and Dr. Massimiliano Copetti of the Biostatistics Unit of Research Institute Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy, for data analysis support.
References
- 1.Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 2.Muchiteni T, Borden WB. Improving risk factor modification: a global approach. Curr Cardiol Rep. 2009;11:476–483. doi: 10.1007/s11886-009-0068-y. [DOI] [PubMed] [Google Scholar]
- 3.Modig K, Andersson T, Drefahl S, et al. Age-specific trends in morbidity, mortality and case-fatality from cardiovascular disease, myocardial infarction and stroke in advanced age: evaluation in the Swedish population. PLoS One. 2013;8:e64928. doi: 10.1371/journal.pone.0064928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617–670. doi: 10.2147/VHRM.S37119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagliardi AC, Miname MH, Santos RD. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis. 2009;202:11–17. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Short RA, Johnson RJ, Tuttle KR. Uric acid, microalbuminuria and cardiovascular events in high-risk patients. Am J Nephrol. 2005;25:36–44. doi: 10.1159/000084073. [DOI] [PubMed] [Google Scholar]
- 7.Glantzounis GK, Tsimoyiannis EC, Kappas AM, et al. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 8.Heinig M, Johnson RJ. Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med. 2006;73:1059–1064. doi: 10.3949/ccjm.73.12.1059. [DOI] [PubMed] [Google Scholar]
- 9.Gabiti-Rosei E, Grassi G. Beyond gout: uric acid and cardiovascular diseases. Curr Med Res Opin. 2013;29(Suppl 3):S33–S39. doi: 10.1185/03007995.2013.790804. [DOI] [PubMed] [Google Scholar]
- 10.Erdogan D, Gullu H, Caliskan M, et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005;59:1276–1282. doi: 10.1111/j.1742-1241.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 11.Strasak AM, Rapp K, Hilbe W, et al. The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28 000 older Austrian women. Ann Oncol. 2007;18:1893–1897. doi: 10.1093/annonc/mdm338. [DOI] [PubMed] [Google Scholar]
- 12.Shin HS, Lee HR, Lee DC, et al. Uric acid as a prognostic factor for survival time: a prospective cohort study of terminally ill cancer patients. J Pain Symptom Manage. 2006;31:493–501. doi: 10.1016/j.jpainsymman.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Sivera F, Andres M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73:328–335. doi: 10.1136/annrheumdis-2013-203325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamanaka H, Metabolism TG. Essence of the revised guideline for the management of hyperuricemia and gout. Japan Med Assoc J. 2012;55:324–329. [PubMed] [Google Scholar]
- 15.Desideri G, Castaldo G, Lombardi A, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. 2014;18:1295–1306. [PubMed] [Google Scholar]
- 16.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puddu PE, Lanti M, Menotti A, et al. Serum uric acid for short-term prediction of cardiovascular disease incidence in the Gubbio population Study. Acta Cardiol. 2001;56:243–251. doi: 10.2143/AC.56.4.2005651. [DOI] [PubMed] [Google Scholar]
- 18.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 19.Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 20.Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17:409–414. doi: 10.1016/j.numecd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Strasak A, Ruttmann E, Brant L, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin Chem. 2008;54:273–284. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 22.Iribarren C, Folsom AR, Eckfeldt JH, et al. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC Study. Atherosclerosis Risk in Communities. Ann Epidemiol. 1996;6:331–340. doi: 10.1016/s1047-2797(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson D, Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013;14:164. doi: 10.1186/1471-2369-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fini MA, Elias A, Johnson RJ, et al. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1:16. doi: 10.1186/2001-1326-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 26.Cicero AF, Salvi P, D'Addato S, et al. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens. 2014;32:57–64. doi: 10.1097/HJH.0b013e328365b916. [DOI] [PubMed] [Google Scholar]