Abstract
Summary
The MOVEST study evaluated the efficacy and safety of monthly oral ibandronate versus licensed monthly IV ibandronate in Japanese osteoporotic patients. Relative BMD gains after 12 months were 5.22 % oral and 5.34 % IV, showing non-inferiority of oral to IV ibandronate (primary endpoint). No new safety concerns were identified.
Introduction
The randomized, phase 3, double-blind MOVEST (Monthly Oral VErsus intravenouS ibandronaTe) study evaluated the efficacy and safety of monthly oral ibandronate versus the licensed monthly intravenous (IV) ibandronate regimen in Japanese patients with osteoporosis.
Methods
Ambulatory patients aged ≥55 years with primary osteoporosis were randomized to receive oral ibandronate 100 mg/month plus monthly IV placebo, or IV ibandronate 1 mg/month plus monthly oral placebo. The primary endpoint was non-inferiority of oral versus IV ibandronate with respect to bone mineral density (BMD) gains at the lumbar spine after 12 months of treatment.
Results
Four hundred twenty-two patients were enrolled with 372 patients in the per-protocol set (183 and 189 in the oral and IV ibandronate groups, respectively). The relative change from baseline in lumbar spine BMD values for the oral and IV ibandronate groups, respectively, was 5.22 % (95 % confidence interval [CI] 4.65, 5.80) and 5.34 % (95 % CI 4.78, 5.90). The least squares mean difference between the two groups was −0.23 % (95 % CI −0.97, 0.51), showing non-inferiority of oral ibandronate to IV ibandronate (non-inferiority limit = −1.60). Changes in BMD values at other sites, and bone turnover marker levels in the oral ibandronate group, were comparable with those of the IV group. The safety profile was similar to that previously demonstrated; no new safety concerns were identified.
Conclusions
This study demonstrated the non-inferiority of oral ibandronate 100 mg/month to IV ibandronate 1 mg/month (licensed dose in Japan) in increasing lumbar spine BMD in Japanese patients with primary osteoporosis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-015-3175-1) contains supplementary material, which is available to authorized users.
Keywords: Ibandronate, MOVEST study, Oral dosing, Osteoporosis
Introduction
The efficacy of ibandronate as a daily treatment regimen was initially demonstrated in the BONE study (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) [1]. Monthly and quarterly dosing regimens of bisphosphonates were subsequently developed with the aim of maintaining adherence and improving outcomes. Based on the bridging strategy, and evidence of an association between bone mineral density (BMD) increases and anti-fracture efficacy [2], the MOBILE (Monthly Oral iBandronate In LadiEs) and DIVA (Dosing IntraVenous Administration) registration trials were conducted and showed superior BMD increases with monthly oral and quarterly intravenous (IV) ibandronate, respectively, versus the daily oral regimen [3–5]. Increases in BMD at all sites were maintained in long-term extensions of these two trials [6, 7].
Meta-analyses of these registration trials confirmed the significant efficacy of monthly oral and quarterly IV regimens of ibandronate in risk reduction of not only vertebral fractures but also non-vertebral and clinical fractures [8, 9]. In addition, a post hoc analysis of individual patient data from the MOBILE and DIVA studies, plus the long-term extensions, revealed a significantly longer time to fracture with intermittent regimens of ibandronate than with placebo over 5 years [10]. These analyses indicated that ibandronate regimens with an annual cumulative exposure (ACE) ≥10.8 mg (i.e., monthly oral 150 mg, quarterly IV 3 mg/3 months) significantly reduced the risk of non-vertebral fractures, including hip fractures, compared with ibandronate regimens with low ACE or placebo.
In Japan, the development of IV regimens of ibandronate was prioritized and the ACE concept was considered. The phase 3 MOVER (MOnthly intraVenous ibandronatE versus daily oral Risedronate) study, conducted for registration purposes in Japan, was designed to demonstrate the non-inferiority of IV ibandronate 1 mg/month (ACE of 12.0 mg) to oral risedronate in vertebral fracture risk reduction. Results of the study showed that IV ibandronate 1 mg/month consistently reduced the incidence of not only vertebral fractures but also non-vertebral fractures, compared with risedronate [11]. Conversely, the optimal dose of monthly oral ibandronate in Japanese osteoporotic patients has not yet been determined. However, the oral 150 mg/month dose is widely used in Western countries with Caucasian (or non-Asian) patients with osteoporosis. The licensed oral doses of alendronate and risedronate for the treatment of osteoporosis in Japan are half those given in Western countries [12–17], whereas the results of the phase 2 dose-finding study with monthly oral ibandronate showed that 100 mg was considered to be the optimal dose for Japanese patients [18].
The purpose of the current registration study was to examine the efficacy and safety of monthly oral ibandronate 100 mg for Japanese patients with osteoporosis and to demonstrate its non-inferiority versus monthly IV ibandronate 1 mg in terms of BMD increases after 12 months of treatment.
Materials and methods
Study design
The MOVEST (Monthly Oral VErsus intravenouS ibandronaTe) study was a prospective, multicenter, randomized, double-blinded, double-dummy comparative study that compared oral ibandronate 100 mg/month with IV ibandronate 1 mg/month in Japanese women and men with osteoporosis (Clinical trial number JapicCTI-121982). The primary endpoint was the non-inferiority of oral ibandronate versus IV ibandronate with respect to lumbar spine BMD (L2–L4) gains after 12 months of treatment.
Patients
Japanese women and men aged ≥55 years with primary osteoporosis according to the Diagnosis Criteria of Primary Osteoporosis in Japan [19] were eligible. Patients were required to have BMD of the lumbar spine (L2–L4) <70 % of the young adult mean (T score less than −2.6 standard deviations [SD]) or BMD of the lumbar spine (L2–L4) <80 % of the YAM (T score less than −1.7 SD) with fragile bone fracture (non-traumatic osteoporotic fracture that occurred by slight external force combined with low BMD).
Exclusion criteria included vertebral deformations likely to affect lumbar spine (L2–L4) BMD measurements by dual-energy x-ray absorptiometry (DXA) scan in central review; previous radiotherapy of the thoracic spine/lumbar spine/pelvis; secondary osteoporosis or a disease causing decrease in bone volume; a disorder delaying the passage of food through the esophagus; received/planned invasive dental procedures; bisphosphonate use within 1 year of the start of the study, anti-receptor activator of nuclear factor-κB ligand (RANKL) antibody (AMG162) use within 2 years of the start of the study, or prior treatment with ibandronate, zoledronate, cathepsin K inhibitor, anti-sclerostin antibody, parathyroid hormone, or strontium; receipt of drugs likely to affect bone metabolism within 8 weeks of the start of the study; severe cardiac, renal, or hepatic disease; calcium outside the criteria value (<8.4 or >10.4 mg/dL); hypersensitivity to bisphosphonate, calcium, or vitamin D; and active malignant tumor or prior therapy for malignant tumor within 3 years.
Treatment
Patients were randomly assigned to receive 100 mg/month oral ibandronate (F. Hoffmann-La Roche, Ltd.) plus IV placebo, or 1 mg/month IV ibandronate (F. Hoffmann-La Roche, Ltd.) plus oral placebo for 12 months by the double dummy method. Patients were instructed to take ibandronate or placebo tablets 60 min before their first food or drink of the day, swallowed whole with a full glass of water while sitting or standing in an upright position. All patients received supplementary calcium 610 mg and vitamin D 400 IU/day from the registration date until the end of the study. Study drug administration was recorded by the investigator at the time of dosing.
Randomization and blinding
Randomization was performed centrally through dynamic allocation (minimization method) based on the patient’s L2–L4 BMD value at screening (T-score <−3.0 vs. ≥−3.0). Patients, investigators, steering committee members, the sponsor, and the faculty who adjudicated the study endpoints remained unaware of treatment-group assignments throughout the trial.
Study endpoints
The primary endpoint was the percentage change from baseline in lumbar spine (L2–L4) BMD at 12 months. Secondary endpoints were the percentage change from baseline in total hip, femoral neck, and trochanter BMD; change from baseline in bone turnover markers (BTMs) of urinary C-telopeptide of type 1 collagen corrected by creatinine (uCTX), serum tartrate-resistant acid phosphatase 5b (TRAP 5b), urinary N-telopeptide of type 1 collagen corrected by creatinine (uNTX), serum procollagen type 1N-terminal propeptide (P1NP), and serum bone-specific alkaline phosphatase (BALP); incidences of non-traumatic new vertebral or non-vertebral fractures; and safety.
Schedule of assessments
BMD measurements at the lumbar spine (L2–L4), total hip, femoral neck, and trochanter were performed centrally (BioClinica, Newark, CA, USA) at screening, baseline, 4, 6, and 12 months using DXA of Hologic bone densitometers. Measurements of a quality control phantom were collected and analyzed by BioClinica to monitor the stability of each DXA scanner. Each study site received the cross-calibration phantom and cross-calibration scans were sent to BioClinica for processing and statistical analysis. BTMs were measured centrally (LSI Medience Corporation, Tokyo, Japan) at baseline, 1, 3, 6, and 12 months. All samples were obtained under fasting conditions prior to study drug administration.
Radiographs of the thoracic and lumbar spine were taken at screening, baseline, and at 12 months after treatment for the assessment of fracture incidence. To identify morphometric vertebral fractures, the vertebral bodies of the lateral projection from Th4 to L4 were assessed using semiquantitative (SQ) methodology by a central committee who were blinded to treatment. A vertebral fracture was defined as an increase of ≥1 SQ grading scale in a vertebra from baseline. Radiographs were assessed by investigators to identify non-vertebral fractures in patients with clinical symptoms.
Adverse events (AEs) were collected throughout the study period and for up to 28 days after the end of treatment. AEs of interest such as renal, cardiac, and gastrointestinal (GI) disorders, esophageal irritation, acute phase reactions (APRs; occurring within 3 days of dosing and lasting for no longer than 7 days), hypocalcemia, osteonecrosis of the jaw, and atypical fracture of the femur were specified in advance.
Statistical analyses
The primary analysis was performed on the per-protocol set (PPS). Analyses of BMD and BTMs were analyzed on the relative change from baseline. Missing data were imputed by the last observation carried forward method. An analysis of covariance was used for the comparison of the relative change in BMD gains at the lumbar spine at 12 months between the two treatment groups with covariates as the BMD and P1NP values at baseline, interaction of BMD values at baseline, prior therapy with bisphosphonates and other osteoporotic agents except bisphosphonates. The covariates, except BMD values at baseline, were added based on a blind review of data. The non-inferiority limit for oral ibandronate to IV ibandronate was set at −1.6. If the lower limit of the 95 % confidence interval (CI) of the least squares mean difference was above the non-inferiority limit, the non-inferiority of oral ibandronate to IV ibandronate would be concluded.
Based on analyses in the MOVER study [11], the relative change in lumbar spine BMD (L2–L4) between oral and IV ibandronate after 12 months of treatment was estimated to be comparable and its SD was estimated to be 4.5 %. The non-inferiority margin was set within 1.6 %, with a one-sided significance level of 0.05 and a detection power of 90 %. With an expected drop-out rate of 15 %, 168 patients were required in each treatment group for analysis, and 198 patients were targeted for registration purposes.
Results
Patient disposition and baseline characteristics
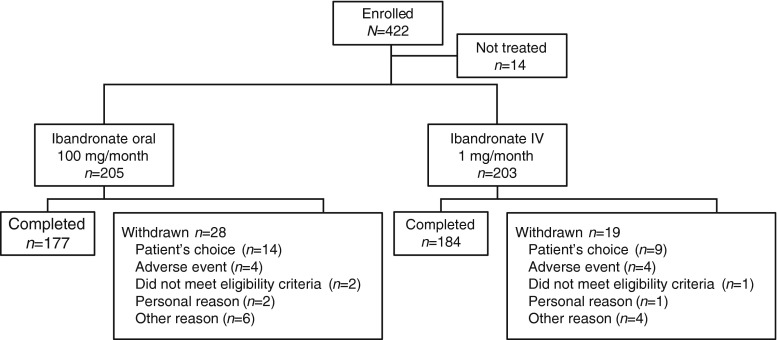
A total of 422 patients were enrolled. Fourteen patients did not receive any study drug, leaving 205 and 203 patients (198 and 199 women) in the safety population who were randomized to receive oral ibandronate 100 mg/month and IV ibandronate 1 mg/month, respectively (Fig. 1). Overall, 177 and 184 patients in the oral and IV ibandronate groups, respectively, completed the study. The PPS for the primary endpoint analysis comprised 183 and 189 patients in the oral 100 mg/month and IV 1 mg/month groups, respectively. Baseline patients’ characteristics between the two treatment groups were well balanced (Table 1).
Fig. 1.
Patient flow through the study. IV intravenous
Table 1.
Baseline patient characteristics
| Characteristic | Ibandronate | |
|---|---|---|
| Oral 100 mg/month (n = 183) | IV 1 mg/month (n = 189) | |
| Women, n (%) | 177 (96.7) | 186 (98.4) |
| Age, years (SD) | 68.8 (6.94) | 69.3 (6.02) |
| 55–74 years, n (%) | 138 (75.4) | 156 (82.5) |
| ≥75 years, n (%) | 45 (24.6) | 33 (17.5) |
| Weight, kg (SD) | 49.5 (7.2) | 49.2 (6.7) |
| Height, cm (SD) | 152.2 (6.5) | 151.6 (6.1) |
| BMD T-score (SD) | ||
| Lumbar spine (L2–L4) | −3.09 (0.58) | −3.14 (0.60) |
| Total hip | −2.41 (0.84) | −2.47 (0.79) |
| Femoral neck | −2.98 (0.82) | −2.99 (0.78) |
| Prevalent vertebral fractures, n (%) | ||
| 0 | 124 (67.8) | 130 (68.8) |
| 1 | 34 (18.6) | 34 (18.0) |
| ≥2 | 25 (13.7) | 25 (13.2) |
| uCTX, μg/mmol CR (SD) | 247.9 (138.8) | 249.4 (166.4) |
| TRAP 5b, mU/dL (SD) | 387.4 (131.6) | 389.2 (152.8) |
| P1NP, μg/L (SD) | 50.6 (21.36) | 49.0 (22.30) |
| BALP, μg/L (SD) | 17.1 (6.78) | 16.5 (6.91) |
| 25-OH(D), ng/mL (SD) | 25.3 (6.26) | 25.3 (5.84) |
Values are the mean, except where indicated
BALP bone-specific alkaline phosphatase, BMD bone mineral density, CR creatinine, IV intravenous, P1NP procollagen type 1N-terminal propeptide, SD standard deviation, TRAP 5b tartrate-resistant acid phosphatase 5b, uCTX creatinine-corrected urinary collagen type 1 cross-linked C-telopeptide, 25-OH(D) 25-hydroxyvitamin D
Bone mineral density
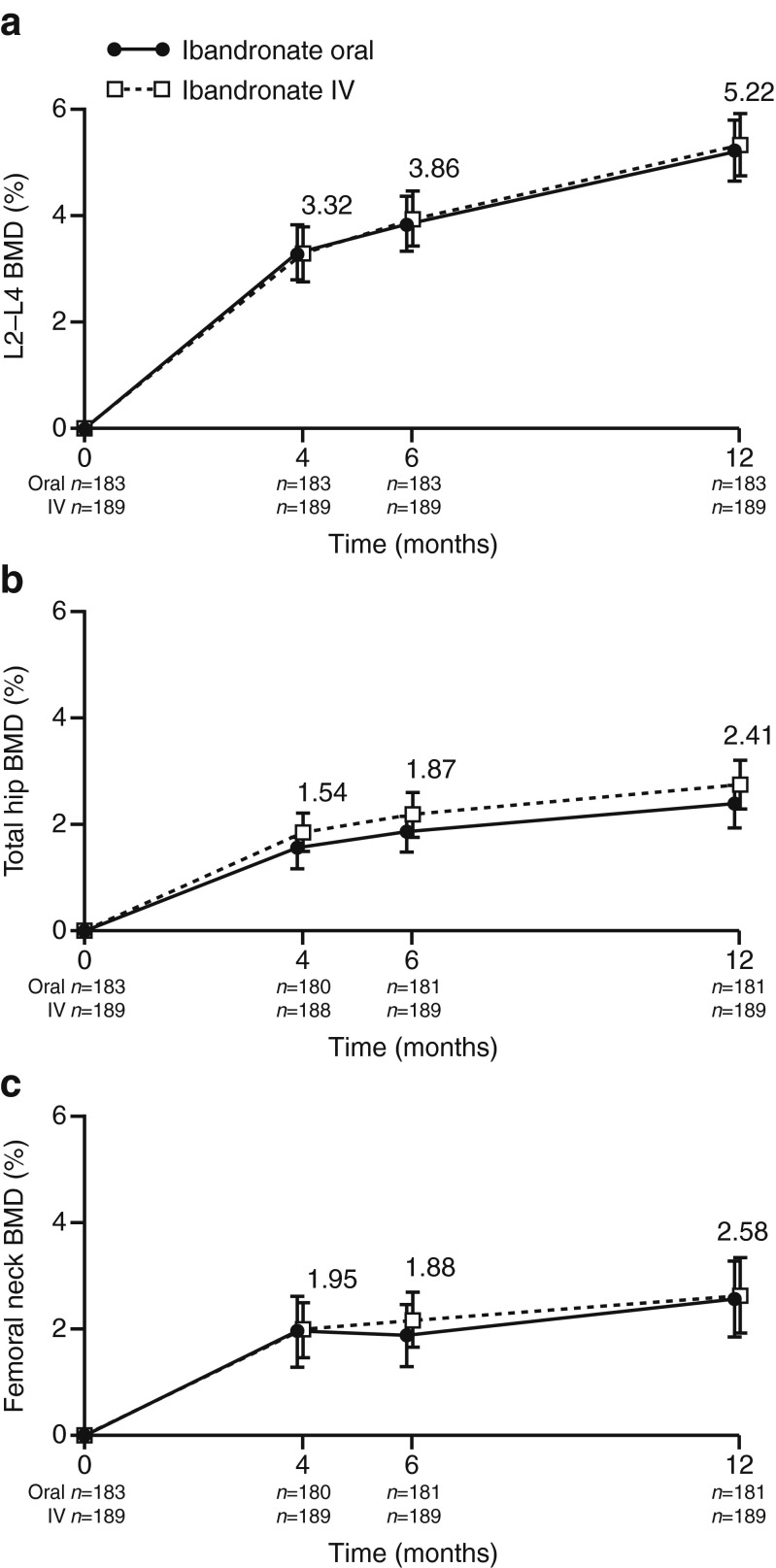
Similar BMD gains were observed with the two treatment regimens throughout the study. The mean relative change from baseline in lumbar spine BMD at 12 months was 5.22 % (95 % CI 4.65, 5.80) and 5.34 % (95 % CI 4.78, 5.90) with oral and IV ibandronate, respectively (Fig. 2a). The least squares mean difference between the two groups was −0.23 % (95 % CI −0.97, 0.51), showing the non-inferiority of oral ibandronate 100 mg/month to IV ibandronate 1 mg/month (non-inferiority limit = −1.60). Sensitivity analysis showed that the result of the primary analysis was robust with or without the added covariates (data not shown). Mean relative changes from baseline to 12 months in total hip BMD were 2.41 % (95 % CI 1.95, 2.87) with oral ibandronate and 2.76 % (95 % CI 2.33, 3.19) with IV ibandronate (Fig. 2b), and in femoral neck BMD were 2.58 % (95 % CI 1.87, 3.29) and 2.64 % (95 % CI 2.06, 3.23), respectively (Fig. 2c). BMD responder rates at the lumbar spine, total hip, and femoral neck were consistent between the two groups after 12 months of treatment (Table 2).
Fig. 2.
Mean relative change from baseline to 12 months (with 95 % CI) in BMD at the a lumbar spine (L2–L4), b total hip, and c femoral neck. BMD bone mineral density, CI confidence interval, IV intravenous
Table 2.
Responder rates (with 95 % CI) after 12 months of treatment
| Responder ratea (%) | Ibandronate | |
|---|---|---|
| Oral 100 mg/month | IV 1 mg/month | |
| Patients with >0 % increase in BMD | ||
| L2–L4 | 91.8 (86.8, 95.3) | 92.1 (87.2, 95.5) |
| Total hip | 86.2 (80.3, 90.9) | 91.5 (86.6, 95.1) |
| Femoral neck | 71.3 (64.1, 77.7) | 74.1 (67.2, 80.2) |
| Patients with ≥3 % increase in BMD | ||
| L2–L4 | 71.6 (64.5, 78.0) | 75.7 (68.9, 81.6) |
| Total hip | 39.2 (32.1, 46.7) | 43.4 (36.2, 50.8) |
| Femoral neck | 43.1 (35.8, 50.6) | 41.8 (34.7, 49.2) |
BMD bone mineral density, CI confidence interval, IV intravenous
aDefined as the proportion of patients with mean lumbar spine (L2–L4), total hip, or femoral neck BMD above baseline
Bone turnover markers
The mean relative change from baseline in uCTX, serum TRAP 5b, and uNTX levels as bone resorption markers, and in serum P1NP and serum BALP, was similar throughout 12 months of oral and IV ibandronate treatment (Fig. 3, uNTX data not shown). The decrease from baseline in uCTX levels at 12 months was 62.80 % (95 % CI 56.62, 68.97) with oral ibandronate and 59.51 % (95 % CI 53.70, 65.33) with IV ibandronate (Fig. 3a). The decrease in TRAP 5b levels was 46.42 % (95 % CI 43.99, 48.85) and 44.65 % (95 % CI 41.84, 47.45), respectively (Fig. 3b). Decreases in serum P1NP were 68.98 % (95 % CI 66.95, 71.02) and 66.66 % (95 % CI 63.45, 69.87), respectively, with oral and IV ibandronate (Fig. 3c), and in serum BALP were 47.28 % (95 % CI 45.03, 49.53) and 43.35 % (95 % CI 40.30, 46.40), respectively (Fig. 3d).
Fig. 3.
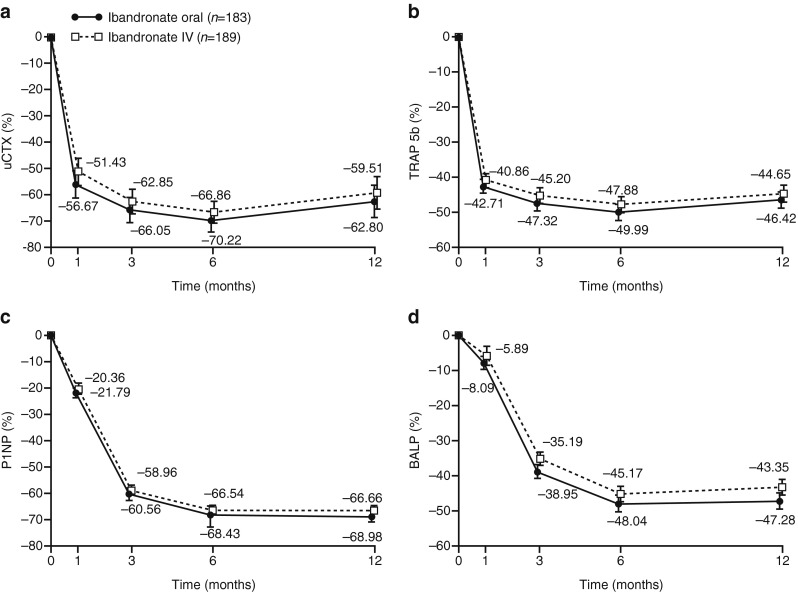
Mean relative change from baseline to 12 months (with 95 % CI) in a uCTX, b serum TRAP 5b, c serum P1NP, and d serum BALP. BALP bone-specific alkaline phosphatase, CI confidence interval, IV intravenous, P1NP procollagen type 1N-terminal propeptide, TRAP 5b tartrate-resistant acid phosphatase 5b, uCTX creatinine-corrected urinary collagen type 1 cross-linked C-telopeptide
Fractures
No differences in fracture incidence were observed between the two treatment groups. The incidence of vertebral fracture over 12 months was 1.1 % (2/183) with oral ibandronate and 0.5 % (1/189) with IV ibandronate. The cumulative incidences of non-vertebral fractures over 12 months were 1.1 % (2/183) and 2.6 % (5/189), respectively.
Adverse events
No apparent differences were observed between the two treatment groups with respect to the incidence of all AEs, all drug-related AEs, or AEs leading to withdrawal (Table 3). The incidence of GI-related AEs was 12.2 and 9.9 % in the oral and IV ibandronate groups, respectively. The incidence of APRs was also similar between the two groups: 11.2 and 11.8 % in the oral and IV ibandronate groups, respectively. However, APR AEs in the oral ibandronate group were mild in intensity, except for three moderate cases (one bone pain and two APR as preferred term), and all APR AEs in the IV ibandronate group were mild. In both treatment groups, APR AEs were transient and decreased with each subsequent dose of medication (Supplementary Fig. 1). As AEs of special interest, the incidence of esophageal irritation-related AEs was 1.0 and 2.5 % with oral and IV ibandronate, respectively. There were no renal function-related AEs, hypocalcemia, osteonecrosis of the jaw, or atypical fracture of the femur (Table 3).
Table 3.
Summary of adverse events
| AE, n (%) | Ibandronate | |
|---|---|---|
| Oral 100 mg/month (n = 205) | IV 1 mg/month (n = 203) | |
| Any AE | 175 (85.4) | 177 (87.2) |
| Drug-related AE | 47 (22.9) | 38 (18.7) |
| Severe intensity AE | 2 (1.0) | 0 |
| Serious AE | 9 (4.4) | 6 (3.0) |
| AEs leading to death | 0 | 0 |
| AEs leading to treatment withdrawal | 4 (2.0) | 4 (2.0) |
| AEs occurring in ≥5 % of patients in either group | ||
| Nasopharyngitis | 48 (23.4) | 62 (30.5) |
| Back pain | 22 (10.7) | 24 (11.8) |
| Contusion | 17 (8.3) | 13 (6.4) |
| Osteoarthritis | 12 (5.9) | 4 (2.0) |
| Muscle pain | 4 (2.0) | 11 (5.4) |
| AEs of special interest | ||
| GI related | 25 (12.2) | 20 (9.9) |
| Esophageal irritation | 2 (1.0) | 5 (2.5) |
| APRa related | 23 (11.2) | 24 (11.8) |
| Back pain | 7 (3.4) | 6 (3.0) |
| APR | 5 (2.4) | 4 (2.0) |
| Malaise | 5 (2.4) | 2 (1.0) |
| Arthralgia | 2 (1.0) | 4 (2.0) |
| Myalgia | 0 | 4 (2.0) |
| Renal function related | 0 | 0 |
| Hypocalcemia | 0 | 0 |
| Osteonecrosis of the jawb | 0 | 0 |
| Atypical fracture of the femurb | 0 | 0 |
AE adverse event, APR acute phase reaction, GI gastrointestinal, IV intravenous
aOccurring within 3 days of dosing and lasting for no longer than 7 days; APR AEs occurring at an incidence of ≥2 % of patients in either treatment group are listed
bAs per the American Society of Bone and Mineral Research case definition
Discussion
We compared the efficacy and safety of oral 100 mg/month and IV 1 mg/month ibandronate in terms of changes in BMD and BTMs in Japanese patients with primary osteoporosis for registration purposes. Oral ibandronate was non-inferior to IV ibandronate with respect to lumbar spine BMD gains after 12 months of treatment. BMD gains at femur sites and BTM suppression with oral ibandronate were consistent with those of IV ibandronate. The safety profile of oral ibandronate was comparable not only with that of IV ibandronate but also with that of previously examined intermittent ibandronate regimens [1, 3, 6]. The oral ibandronate regimen was well tolerated.
BMD gains have been recognized as a surrogate marker for future fracture risk. The relationship between increases in BMD and fracture risk reduction by treatment with ibandronate has been reported previously [20, 21]. Patients receiving ibandronate at high ACE (10.8–12.0 mg) showed higher BMD increases and stronger anti-fracture efficacy than those treated at low ACE (5.5 mg) [9]. In the MOVER study, IV ibandronate 1 mg/month (ACE of 12.0 mg) demonstrated non-inferiority to risedronate in terms of vertebral fracture risk reduction [11]. The fact that the oral ibandronate 100 mg/month regimen was bridged to the IV 1 mg/month regimen in this study suggests that monthly oral ibandronate 100 mg would be efficacious in fracture risk reduction.
Nakai et al. recently reported that the area under the ibandronate serum-concentration time curve (AUC) and the relative change in uCTX levels with oral ibandronate 100 mg/month were comparable with those of IV ibandronate 1 mg/month in Japanese postmenopausal women [22]. The optimal monthly dose of ibandronate for Japanese patients was confirmed as 100 mg, based on the results of the phase 2 dose-finding study [18] and the current pivotal bridging clinical trial. The optimal monthly dose for Caucasian patients in Western countries is 150 mg, which is higher than that for Japanese patients. The bioavailability of oral ibandronate is reported to be 0.63 % in Western populations [23] and 0.91 % in Japanese populations [22]. This 1.44-times higher bioavailability in Japanese patients could lead to a 1.5-times higher optimal dose in Western patients. In fact, the bioavailability of bisphosphonates, including risedronate, in Japanese patients has been reported to be slightly higher than in Western populations [24–26]. The reason for this difference in bioavailability is still unknown [22]. Overall, exposure to ibandronate is similar in Japanese and Western populations because of dose optimization in the different settings. This suggests that oral ibandronate, when dosed optimally, offers similar efficacy and safety benefits to Japanese and Western patients.
The safety profile of ibandronate in this current study was similar to previous studies in Western patients with no apparent increase in the nature or severity of AEs. APR is commonly experienced following the first administration of nitrogen-containing bisphosphonates. The incidence of APR AEs was similar with monthly oral and IV ibandronate, administered at comparable ACE, and they were reported at a similar frequency to previous studies [3, 4, 11]. The incidence of GI-related AEs was also similar between the treatment groups. Thus, oral ibandronate 100 mg/month appears to be well tolerated by Japanese osteoporotic patients with a similar safety profile to the established monthly oral regimen in Western populations.
There are some limitations to this study. Firstly, regarding concerns about GI-related AEs with oral bisphosphonate treatment, the incidence of these AEs in the current study may not reflect the effects seen in daily practice due to the double dummy design used. In addition, we assessed fracture incidence over 12 months of treatment, but there are as yet limited data on the fracture incidence with 100 mg/month ibandronate in Japanese patients over longer treatment periods. Further clinical evidence must be accumulated on long-term treatment duration in this patient population.
Better adherence to treatment can lead to improved outcomes in patients with osteoporosis, both in terms of BMD gains and in fracture risk reduction. Although bisphosphonates have been used as first-line therapy for many years, their complex dosing instructions have resulted in adherence issues with long-term treatment. Adherence is higher with weekly rather than daily bisphosphonates [27] and with monthly rather than weekly treatment [28, 29]. A preference for monthly oral bisphosphonates has also been reported [30, 31]. These findings suggest that the availability of a monthly oral ibandronate dosing regimen would create an opportunity to improve adherence in Japanese patients with osteoporosis.
In conclusion, oral ibandronate 100 mg/month demonstrated non-inferiority to IV ibandronate 1 mg/month (licensed dose in Japan) in lumbar spine BMD gains in Japanese patients with primary osteoporosis. The safety profile of oral and IV ibandronate was similar, and monthly oral ibandronate was as well tolerated in Japanese patients as in Western patients. These data suggest that these two different monthly ibandronate regimens are effective alternatives for the treatment of osteoporosis in Japanese patients.
Electronic supplementary material
Change in the incidence of acute phase reactions between first and subsequent dose of study medication. IV intravenous (GIF 15 kb)
Acknowledgments
The authors would like to acknowledge the other members of the MOVEST study group: Satoru Nakajo, Yu Miyazaki, Tetsuro Nakamura, Atsuko Abe, Masaharu Shiraishi, Akihito Tomonaga, Masako Aso, Satoshi Ikeda, Yasuo Imanishi, Takafumi Inoue, Hiroshi Tsurukami, Sadafumi Kato, Yutaka Suzuki, Akira Takiguchi, Hiroaki Shibata, Kimiyasu Ishikawa, Michio Yagi, Yasuaki Miki, Yasuo Ito, Yoshio Fujii, Takayoshi Suga, and Keisuke Goto. The MOVEST study was jointly designed by the primary investigators and the sponsors, Chugai Pharmaceutical Co. Ltd. and Taisho Pharmaceutical Co. Ltd. Data management, quality control, and all analyses for publication were the responsibility of Chugai Pharmaceutical Co. Ltd. All authors contributed to the manuscript and have approved the final version for submission. The authors acknowledge Dr. Daiva Masanauskaite, Dr. Cornelis Schep, and Dr. Joseph Kohles of F. Hoffmann-La Roche Ltd. for discussing the results with them. Support for third-party writing assistance for this manuscript was provided by Chugai Pharmaceutical Co. Ltd.
Conflicts of interest
T. Nakamura has received research grants and/or consulting fees from Asahi Kasei Pharma Corp., Astellas Pharma Inc., Banyu Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Inc., Eisai Co. Ltd., Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Teijin Pharma Ltd., and belongs to the Japan Ministry of Health, Welfare and Labor as a councilor for hospital administration and social medical insurance. M. Ito has received consulting fees from Chugai Pharmaceutical Co. Ltd. and consulting fees from Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Inc., and Ono Pharmaceutical Co. Ltd. J. Hashimoto, K. Shinomiya, Y. Asao, and K Katsumata are employees of Chugai Pharmaceutical Co. Ltd. H. Hagino has received consulting fees from Asahi Kasei Pharma Corp., Astellas Pharma Inc., Banyu Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma Corp., Ono Pharmaceutical Co. Ltd., Pfizer Inc., Takeda Pharmaceutical Co. Ltd., and Teijin Pharma Ltd. T. Inoue is an employee of Taisho Pharmaceutical Co. Ltd. T. Nakano has received consulting fees from Asahi Kasei Pharma Corp., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Inc., and Teijin Pharma Ltd. H. Mizunuma has received consulting fees from Chugai Pharmaceutical Co. Ltd. and Serono Japan Co. Ltd.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Chesnut CH, III, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD, Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 2.Leslie WD, Tsang JF, Caetano PA, Lix LM, Manitoba Bone Density Program Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab. 2007;92:77–81. doi: 10.1210/jc.2006-1415. [DOI] [PubMed] [Google Scholar]
- 3.Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, Christiansen C, Rowell L, Mairon N, Bonvoisin B, Drezner MK, Emkey R, Felsenberg D, Cooper C, Delmas PD, Miller PD. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65:654–661. doi: 10.1136/ard.2005.044958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisman JA, Civitelli R, Adami S, Czerwinski E, Recknor C, Prince R, Reginster JY, Zaidi M, Felsenberg D, Hughes C, Mairon N, Masanauskaite D, Reid DM, Delmas PD, Recker RR. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol. 2008;35:488–497. [PubMed] [Google Scholar]
- 5.Delmas PD, Adami S, Strugala C, Stakkestad JA, Reginster JY, Felsenberg D, Christiansen C, Civitelli R, Drezner MK, Recker RR, Bolognese M, Hughes C, Masanauskaite D, Ward P, Sambrook P, Reid DM. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum. 2006;54:1838–1846. doi: 10.1002/art.21918. [DOI] [PubMed] [Google Scholar]
- 6.Miller PD, Recker RR, Reginster JY, Riis BJ, Czerwinski E, Masanauskaite D, Kenwright A, Lorenc R, Stakkestad JA, Lakatos P. Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long-term extension study. Osteoporos Int. 2012;23:1747–1756. doi: 10.1007/s00198-011-1773-0. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi G, Czerwinski E, Kenwright A, Burdeska A, Recker RR, Felsenberg D. Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int. 2012;23:1769–1778. doi: 10.1007/s00198-011-1793-9. [DOI] [PubMed] [Google Scholar]
- 8.Harris ST, Blumentals WA, Miller PD. Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin. 2008;24:237–245. doi: 10.1185/030079908X253717. [DOI] [PubMed] [Google Scholar]
- 9.Cranney A, Wells GA, Yetisir E, Adami S, Cooper C, Delmas PD, Miller PD, Papapoulos S, Reginster JY, Sambrook PN, Silverman S, Siris E, Adachi JD. Ibandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient data. Osteoporos Int. 2009;20:291–297. doi: 10.1007/s00198-008-0653-8. [DOI] [PubMed] [Google Scholar]
- 10.Miller PD, Recker RR, Harris S, Silverman S, Felsenberg D, Reginster J, Day DM, Barr C, Masanauskaite D. Long-term fracture rates seen with continued ibandronate treatment: pooled analysis of DIVA and MOBILE long-term extension studies. Osteoporos Int. 2014;25:349–357. doi: 10.1007/s00198-013-2518-z. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Nakano T, Ito M, Hagino H, Hashimoto J, Tobinai M, Mizunuma H, MOVER Study Group Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int. 2013;93:137–146. doi: 10.1007/s00223-013-9734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, Kaneda K, Minaguchi H, Inoue T, Morii H, Tomita A, Yamamoto K, Nagata Y, Nakashima M, Orimo H. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int. 1999;10:183–192. doi: 10.1007/s001980050214. [DOI] [PubMed] [Google Scholar]
- 13.Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M, Fukunaga M, Tomomitsu T, Ohashi Y, Nakamura T. Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab. 2005;23:382–388. doi: 10.1007/s00774-005-0616-5. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga M, Kushida K, Kishimoto H, Shiraki M, Taketani Y, Minaguchi H, Inoue T, Morita R, Morii H, Yamamoto K, Ohashi Y, Orimo H, Risedronate Phase III Research Group A comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial. Osteoporos Int. 2002;13:971–979. doi: 10.1007/s001980200135. [DOI] [PubMed] [Google Scholar]
- 15.Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A, Inoue T, Kaneda K, Morii H, Nawata H, Yamamoto K, Ohashi Y, Orimo H. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab. 2004;22:469–478. doi: 10.1007/s00774-004-0509-z. [DOI] [PubMed] [Google Scholar]
- 16.Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, Nakamura T, Ohta H, Takaoka K, Ohashi Y, Risedronate Phase III Research Group (2006) Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. J Bone Miner Metab 24:405–413 [DOI] [PubMed]
- 17.Hagino H, Kishimoto H, Ohishi H, Horii S, Nakamura T. Efficacy, tolerability and safety of once-monthly administration of 75 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with a 2.5 mg once-daily dosage regimen. Bone. 2014;59:44–52. doi: 10.1016/j.bone.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Mizunuma H, Itabashi A, Yamane H, Hannita T, Karube M, Takeuchi K, Ikegami R, Okamoto S, Hasunuma T, Kumagai Y. Monthly oral ibandronate is well tolerated and efficacious in Japanese osteoporotic subject. J Bone Miner Res. 2007;22(Suppl 1):S333. [Google Scholar]
- 19.Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H, Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 20.Sebba AI, Emkey RD, Kohles JD, Sambrook PN. Ibandronate dose response is associated with increases in bone mineral density and reductions in clinical fractures: results of a meta-analysis. Bone. 2009;44:423–427. doi: 10.1016/j.bone.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Miller PD, Delmas PD, Huss H, Patel KM, Schimmer RC, Adami S, Recker RR. Increases in hip and spine bone mineral density are predictive for vertebral antifracture efficacy with ibandronate. Calcif Tissue Int. 2010;87:305–313. doi: 10.1007/s00223-010-9403-y. [DOI] [PubMed] [Google Scholar]
- 22.Nakai K, Tobinai M, Hashimoto J, Iida S, Kawanishi T (2014) The optimal oral dose selection of ibandronate in Japanese patients with osteoporosis based on pharmacokinetic and pharmacodynamic properties. Eur J Drug Metab Pharmacokinet doi:10.1007/s13318-014-0242-5 [DOI] [PMC free article] [PubMed]
- 23.Barrett J, Worth E, Bauss F, Epstein S. Ibandronate: a clinical pharmacological and pharmacokinetic update. J Clin Pharmacol. 2004;44:951–965. doi: 10.1177/0091270004267594. [DOI] [PubMed] [Google Scholar]
- 24.Ogura Y, Gonsho A, Cyong JC, Orimo H. Clinical trial of risedronate in Japanese volunteers: single and multiple oral dose studies. J Bone Miner Metab. 2004;22:111–119. doi: 10.1007/s00774-003-0458-y. [DOI] [PubMed] [Google Scholar]
- 25.Shiraki M, Fukunaga M, Kushida K, Kishimoto H, Taketani Y, Minaguchi H, Inoue T, Morita R, Morii H, Yamamoto K, Ohashi Y, Orimo H. A double-blind dose-ranging study of risedronate in Japanese patients with osteoporosis (a study by the Risedronate Late Phase II Research Group) Osteoporos Int. 2003;14:225–234. doi: 10.1007/s00198-002-1369-9. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell DY, Eusebio RA, Sacco-Gibson NA, Pallone KA, Kelly SC, Nesbitt JD, Brezovic CP, Thompson GA, Powell JH. Dose-proportional pharmacokinetics of risedronate on single-dose oral administration to healthy volunteers. J Clin Pharmacol. 2000;40:258–265. doi: 10.1177/00912700022008928. [DOI] [PubMed] [Google Scholar]
- 27.Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21:1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 28.Cotté FE, Fardellone P, Mercier F, Gaudin AF, Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21:145–155. doi: 10.1007/s00198-009-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper A, Drake J, Brankin E, (Fi - this is what's on PubMed and in the original manuscript, so not sure why the journal has flipped the words around) (2006) Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract 60:896–905 [DOI] [PMC free article] [PubMed]
- 30.Emkey R, Koltun W, Beusterien K, Seidman L, Kivitz A, Devas V, Masanauskaite D. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO) Curr Med Res Opin. 2005;21:1895–1903. doi: 10.1185/030079905X74862. [DOI] [PubMed] [Google Scholar]
- 31.Hadji P, Minne H, Pfeifer M, Bourgeois P, Fardellone P, Licata A, Devas V, Masanauskaite D, Barrett-Connor E. Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: a randomized, crossover study (BALTO II) Joint Bone Spine. 2008;75:303–310. doi: 10.1016/j.jbspin.2007.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Change in the incidence of acute phase reactions between first and subsequent dose of study medication. IV intravenous (GIF 15 kb)