Abstract
Key points
Exercise acutely increases the concentrations of metabolites and hormones such as growth hormone (GH) and, to a lesser extent, insulin‐like growth factor 1 (IGF‐1); however, the biological function of this response is unclear.
Pharmacological administration of these hormones stimulates collagen synthesis in muscle and tendon; however, whether the post‐exercise biochemical milieu has a similar action is unknown.
Treating engineered ligaments with serum obtained from young healthy men after exercise resulted in more collagen and improved tensile strength over those treated with serum from resting men.
Further, we show that the increase in collagen induced by post‐exercise serum (i) is not reproduced by treatment with recombinant GH or IGF‐1, and (ii) is associated with the activation of PI3 kinase/mTORC1 and ERK1/2 signalling.
Abstract
Exercise stimulates a dramatic change in the concentration of circulating hormones, such as growth hormone (GH), but the biological functions of this response are unclear. Pharmacological GH administration stimulates collagen synthesis; however, whether the post‐exercise systemic milieu has a similar action is unknown. We aimed to determine whether the collagen content and tensile strength of tissue‐engineered ligaments is enhanced by serum obtained post‐exercise. Primary cells from a human anterior cruciate ligament (ACL) were used to engineer ligament constructs in vitro. Blood obtained from 12 healthy young men 15 min after resistance exercise contained GH concentrations that were ∼7‐fold greater than resting serum (P < 0.001), whereas IGF‐1 was not elevated at this time point (P = 0.21 vs. rest). Ligament constructs were treated for 7 days with medium supplemented with serum obtained at rest (RestTx) or 15 min post‐exercise (ExTx), before tensile testing and collagen content analysis. Compared with RestTx, ExTx enhanced collagen content (+19%; 181 ± 33 vs. 215 ± 40 μg per construct P = 0.001) and ligament mechanical properties – maximal tensile load (+17%, P = 0.03 vs. RestTx) and ultimate tensile strength (+10%, P = 0.15 vs. RestTx). In a separate set of engineered ligaments, recombinant IGF‐1, but not GH, enhanced collagen content and mechanics. Bioassays in 2D culture revealed that acute treatment with post‐exercise serum activated mTORC1 and ERK1/2. In conclusion, the post‐exercise biochemical milieu, but not recombinant GH, enhances collagen content and tensile strength of engineered ligaments, in association with mTORC1 and ERK1/2 activation.
Abbreviations
- ACL
anterior cruciate ligament
- ERK
extracellular signal‐regulated kinase
- GH
growth hormone
- IGF‐1
insulin‐like growth factor 1
- mTORC1
mechanistic/mammalian target of rapamycin complex 1
- TGF‐β1
transforming growth factor‐β1
Introduction
Resistance exercise elevates circulating concentrations of growth hormone (GH) and, to a lesser extent, cortisol, testosterone, and insulin‐like growth factor 1 (IGF‐1; Kraemer et al. 1990). However, the biological roles of the exercise‐induced hormone response are unclear. Whereas the post‐exercise hormone response does not drive resistance exercise‐induced anabolism in skeletal muscle (Wilkinson et al. 2006; West et al. 2009, 2010), other potential biological functions exist. Support for the hormonal shift reflecting a ‘fight or flight’ response stems from the observation that (i) exercise intensity is associated with hormonal rises (Sutton & Lazarus, 1976), (ii) exercise‐induced hormone release can occur in a feed‐forward manner and activate energy‐mobilizing enzymes that do not closely mirror energy needs (Kjaer et al. 1987), and (iii) training can reduce the post‐exercise response of cortisol (West et al. 2010). However, in contrast to training‐induced reductions in the response of the stress hormone cortisol, trained individuals still elicit robust exercise‐induced GH responses (West et al. 2010), suggesting that general stress does not fully explain the change in hormonal milieu. Support for a substrate‐mobilizing role of the exercise‐induced hormone shift stems from the observation that (i) adrenaline activates glycogenolysis during exercise (Spriet et al. 1988; Febbraio et al. 1998), (ii) GH and cortisol induce insulin resistance (i.e. have an ‘anti‐fuel storage’ effect) (Louard et al. 1994), and (iii) GH is gluconeogenic and lipolytic (Moller et al. 1992; Vahl et al. 1997). However, interestingly, GH secretion is also stimulated during sleep (Sutton & Lazarus, 1976; Redwine et al. 2000), a time when energy demands are low and the secretion of other stress‐activated/substrate mobilizing hormones such as cortisol and adrenaline is inhibited (Weitzman et al. 1983; Dodt et al. 1997). Collectively, these observations suggest that there may be other important functions of the rise in circulating factors (metabolites, hormones, growth factors, cytokines) post‐exercise. A third unexplored role for the systemic post‐exercise biochemical shifts (and perhaps exercise‐induced GH per se) is that they might contribute to connective tissue remodelling.
Pharmacologically administered GH increases gene expression as well as synthesis rates of collagen in muscle and tendon (Doessing et al. 2010 a; Boesen et al. 2014). However, assuming that the effects achieved with pharmacological hormone interventions are similar to those achieved as a result of physiological hormonal changes is problematic since hormone concentrations and kinetics can be very different in response to a pharmacological intervention (Schroeder et al. 2013). GH is unique in the outsized nature of its exercise‐induced response. By contrast, exercise‐induced changes in circulating IGF‐1 are equivocal, with increases, decreases and no changes reported (Kraemer et al. 1990, 1991, 1995). Systemic IGF‐1, which is stimulated by pituitary‐released GH and is primarily the result of liver secretions (IGF‐1Ea isoform), is distinct from local compartments of IGF‐1 (overexpression of muscle IGF‐1 (Barton‐Davis et al. 1998) and tendon injection of GH (Vestergaard et al. 2012) do not alter systemic IGF‐1), and does not reflect local IGF‐1 signalling after exercise (Nindl et al. 2012). Further, the activity and half‐life of systemic IGF‐1 is heavily influenced by IGF binding proteins and an acid‐labile subunit (Jones & Clemmons, 1995). Collectively, in the context of the present study, GH and IGF‐1 were of interest a priori due to their well‐characterized regulation of connective tissue turnover (Doessing & Kjaer, 2005; Doessing et al. 2010 a,b; Boesen et al. 2014). Additionally, because hormone responses are complex (e.g. over 100 GH isoforms are reported; Kraemer et al. 2010), it is impossible to recapitulate the physiological response pharmacologically. Therefore, to test the role of the post‐exercise hormonal milieu on connective tissue remodelling, we overlaid a human‐centred approach on an in vitro model of a ligament developed in our laboratory (Paxton et al. 2010 b).
At a mechanistic level, the molecular processes that regulate connective tissue turnover are poorly understood. This is largely due to the difficulty in obtaining human tissue samples as well as the density of the collagen matrix and the small number of cells in the mature tissue. To overcome these difficulties, we developed a three‐dimensional (3D) engineered ligament model that is similar to developing tendon (Kapacee et al. 2008; Paxton et al. 2009, 2010 a,b; Bayer et al. 2010). Like developing tendons/ligaments (Marturano et al. 2014), these engineered tissues have more cells and less matrix, their rate of collagen synthesis is significantly higher (Calve et al. 2010), and they express more developmental collagen isoforms (Bayer et al. 2010) and as a result are much weaker (Paxton et al. 2010 b). In spite of these significant differences, the engineered ligaments respond similarly to native tissue to nutrients (Paxton et al. 2010 b), growth factors (Hagerty et al. 2012), hormones (Lee et al. 2015) and exercise (Mackey et al. 2008; Paxton et al. 2012). Further, engineered ligaments can be mechanically tested to determine their function and therefore might be a good tool for understanding the molecular processes that regulate connective tissue turnover.
The purpose of this study was to determine the effect of the human exercise‐induced biochemical milieu on collagen turnover using ligaments engineered from cells obtained from the human anterior cruciate ligament (ACL). We used a within‐subject design whereby culture medium was enriched with serum obtained at rest and after exercise from the same individual. Thus, the only difference between the two treatments was the serum biochemical milieu to which the engineered ligaments were exposed.
Methods
Participants, blood sampling and exercise
Twelve healthy recreationally active young men (22 ± 2.5 years, 1.79 ± 0.1 m, 80.1 ± 11.5 kg) consented to a protocol that was approved by the Institutional Review Board at University of California Davis and that was written in accordance with standards set by the Declaration of Helsinki. Blood was drawn from an antecubital vein at rest, and 15 min after an acute bout of resistance exercise. After a warm‐up, participants completed a bout of lower body resistance exercise. Exercise consisted of five sets of leg press with 1 min rest intervals between sets, and three sets of knee extensions together with three sets of hamstring curls as a ‘super‐set’ (one set of each exercise back‐to‐back, then 1 min rest). A similar exercise protocol has been used previously (West et al. 2010, 2013) to stimulate an endogenous hormone response. Blood samples were allowed to clot and then centrifuged (1500 g × 10 min), and the resulting sterile serum was collected for preparation of medium for construct treatment. An aliquot of serum was frozen at −80°C for analysis of total GH and IGF‐1 concentrations by ELISA according to the manufacturer's instructions (R&D Systems Inc., Minneapolis, MN, USA).
Engineered ligament formation
Engineered ligaments were formed from primary cells obtained from a human ACL as described previously (Paxton et al. 2010 b). After obtaining informed consent according to a protocol approved by the Institutional Review Board at the University of California Davis, ACL tissue was obtained from a healthy young male donor who was undergoing ACL reconstruction surgery. The tissue was digested overnight in a 0.1% Type II collagenase solution and plated onto 15 cm tissue culture plates. The resulting primary fibroblast population was expanded in growth medium consisting of Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin. Aliquots of the cells were frozen in DMEM containing 20% FBS and 10% dimethyl sulfoxide (DMSO) until needed for experiments. Frozen cells were thawed and expanded by trypsinization to passage 4–5 prior to experimental use. Cells (2.5 × 105) were embedded in a fibrin gel on 35 mm plates containing two cylindrical brushite (calcium phosphate) anchors placed 12 mm apart, used to mimic the bony ends of ligaments and to aid in mechanical testing. Over the next 4 days, the cells contracted the fibrin into a ligament‐like sinew between the two brushite anchors (Paxton et al. 2010 b). Constructs were maintained in feed medium consisting of growth medium supplemented with 5 ng ml−1 TGF‐β1 (Peprotech, Rocky Hill, NJ, USA), 200 μm ascorbic acid and 50 μm proline, which was refreshed every other day.
Ligament construct treatment and analysis
Once fully formed (day 7 after embedding cells), ligament constructs were treated for 7 days with experimental feed medium containing serum obtained at rest or post‐exercise. The treatment was begun at day 7 to give the constructs sufficient time to contract into a linear tissue and eliminate potential effects from the treatment on the formation process. A 7 day treatment regimen was selected to maximize the treatment duration given the amount of serum obtained from each volunteer. All feed media also contained 1% penicillin, 200 μm ascorbic acid and 50 μm proline. Two constructs per condition per serum donor were treated and the average of duplicate constructs was used for all analyses.
We treated according to two schedules, with half of the constructs treated in a continuous fashion, and half treated in a pulsed fashion to mimic the typical transient rise and fall in hormone concentration that occurs in vivo after resistance exercise. Continuous treatment ligaments were maintained in (only) rest or exercise medium, which was refreshed every 24 h. Pulsed‐rest ligaments were exposed to post‐exercise serum for 1 h per day followed by 23 h in rest medium. This pulsed treatment style aimed to broadly reflect the physiological exercise‐induced hormone response. We estimate that the amount of serum we used approximated the in vivo concentration of IGF‐1 and slightly underestimated GH concentration.
To determine the effect of recombinant growth hormone and IGF‐1 on ligament collagen and function, a separate set of engineered ligaments were treated for 7 days with increasing amounts of recombinant human GH (Fig. 3) or IGF‐1 (Fig. 4) (PeproTech, Rocky Hill, NJ, USA) with concentrations noted in the respective figures. The dose of recombinant hormone was designed to encompass both the physiological and a supraphysiological level in an effort to understand how each hormone directly affected the engineered ligaments.
Figure 3. Relationship between growth hormone and ligament collagen content and mechanics .
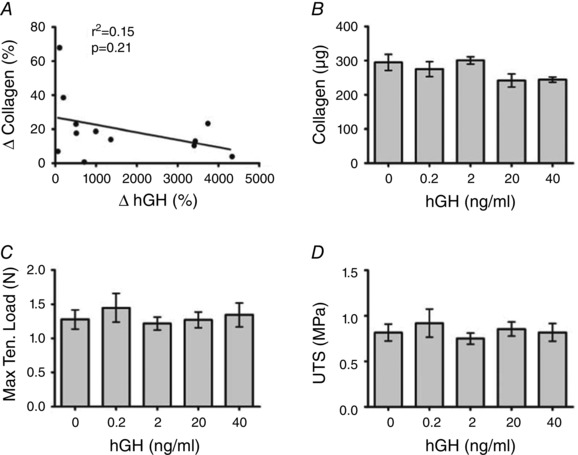
A, the correlation between the change in collagen in ligament constructs (delta of exercise serum treated and rest serum treated) and the change in exercise‐induced growth hormone. B–D, collagen content (B), maximal tensile load (C), and ultimate tensile stress (D) in ligament constructs treated with increasing doses of recombinant human growth hormone. Values are means ± SD with no statistically significant differences. P value and r 2 from linear regression of change in GH versus change in collagen.
Figure 4. Relationship between insulin‐like growth factor and ligament collagen content and mechanics .
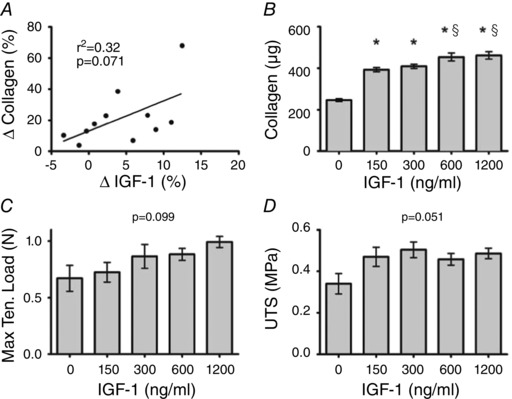
A, the association between the change in collagen in ligament constructs (delta of exercise serum treated and rest serum treated) and the change in exercise‐induced insulin‐like growth factor 1 (IGF‐1). B–D, collagen content (B), maximal tensile load (C) and ultimate tensile stress (D) in ligament constructs treated with increasing doses of recombinant IGF‐1. *Significantly different from control (P < 0.05); §significantly different from 150 and 300 ng ml−1 (P < 0.05). Values are means ± SD. P value and r 2 from linear regression of change in IGF‐1 versus change in collagen.
Ligament construct tensile testing and collagen content analysis
At the end of the 7 day treatment, constructs were tensile‐tested as described previously (Paxton et al. 2010 b). Briefly, the length and cross‐sectional area of the ligaments were determined using digital callipers before the ligament anchors were placed into grips attached to a stepper motor and a force transducer. The ligaments were stretched using a custom LabView program (National Instruments, Austin, TX, USA) at a rate of 0.4 mm s−1 and the resulting force–deformation data were used to calculate maximal tensile load. Using the initial length and cross‐sectional area, we calculated stress and strain and from this information determined the ultimate tensile stress and modulus (stiffness) of the ligaments. Following tensile testing, constructs were dried and weighed, prior to determining collagen content using a hydroxyproline assay as described previously (Paxton et al. 2010 b). Briefly, each sample was hydrolysed in 6 n HCl and dried at 120°C. The resulting pellet was resuspended in hydroxyproline buffer. An aliquot was diluted 1:25 to 200 μl, combined with 150 μl chloramine T solution for 20 min, and then combined with 150 μl aldehyde‐perchloric acid for 15 min at 60°C. Absorbance was read at 550 nm on an Epoch Microplate Spectrophotometer (BioTek Instruments Ltd, Winooski, VT, USA). Hydroxyproline was converted to collagen mass assuming that collagen contains 13.7% hydroxyproline (Creemers et al. 1997). The collagen fraction was determined by dividing the collagen content by the mass of the tissue.
2D fibroblast culture and bioassays
Human ACL fibroblasts were grown to confluence in a monolayer in six‐well plates containing DMEM before acute treatment with (i) increasing doses (concentrations in respective figures) of recombinant human TGF‐β1 (Fig. 5), IGF‐1 (Fig. 6) or GH (Fig. 6), or (ii) serum obtained at rest and after exercise. After washing in ice‐cold PBS, fibroblasts were collected in 100 μl lysis buffer with scraping. For the TGF‐β1 bioassay, cytosolic and nuclear fractions were isolated. Fibroblasts were collected in cytosolic extraction buffer (250 mm sucrose, 50 mm Tris pH 7.4, 5 mm MgCl2, and protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL, USA)) and centrifuged (800 g × 15 min), and supernatants containing cytosolic proteins were collected. Pellets were washed twice in cytosolic extraction buffer before resuspension in nuclear extraction buffer (20 mm Hepes, 1.5 mm MgCl2, 0.5 m NaCl, 0.2 mm EDTA, 20% glycerol, 1% Triton X‐100, protease inhibitors), brief sonication, and centrifugation (11,000 g × 15 min) to obtain supernatants containing nuclear proteins. Cytosolic and nuclear fractions were each mixed with Laemmli sample buffer, heated at 95°C for 5 min and stored at −30°C for subsequent analysis by Western blot as previously described (Baehr et al. 2014). Primary antibodies (Cell Signaling Technology, Danvers, MA, USA) were used to probe the following phosphorylation targets: Smad2/3 Ser423/425, Akt Ser473, S6K1 Thr389, 4EBP1 Thr37/46 and ERK1/2 Thr202/Tyr204. Membranes were incubated in primary antibody overnight before secondary antibody incubation (horseradish peroxidase, Thermo Scientific Pierce) and detection by chemiluminescence (Immobilon HRP substrate, EMD Millipore Corp.). Images were captured using a ChemiDoc MP system and densitometry was quantified using Image Lab 5.0 software (Bio‐Rad Laboratories Inc., Hercules, CA, USA).
Figure 5. Nuclear Smad levels following treatment with TGF‐β1 or exercise serum .
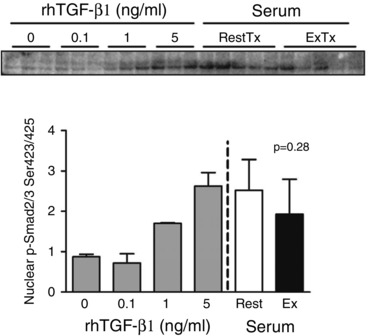
A, nuclear abundance of phospho‐Smad2/3 in human fibroblasts after a 1 h treatment with increasing doses of recombinant TGF‐β1 or serum (10% v/v) from participants obtained at rest or 15 min after resistance exercise. P value reflects the difference between rest and exercise serum treatments. Values are means ± SD.
Figure 6. Post‐exercise serum activates PI3‐kinase/mTORC1 and ERK1/2 signalling .
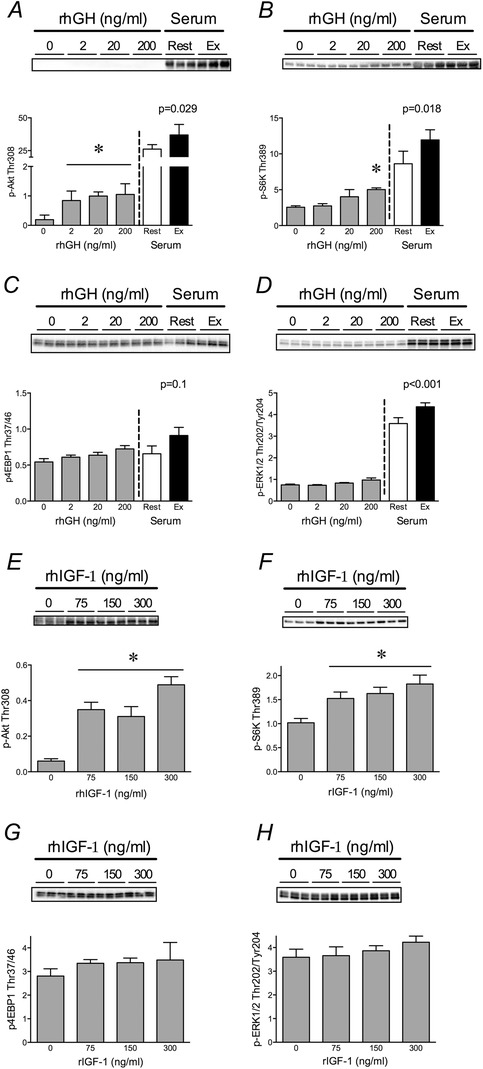
A–D, phosphorylation of pAkt Thr308 (A), S6K1 Thr389 (B), 4E‐BP1 Thr 37/46 (C), and Erk1/2 Thr202/Tyr204 (D) in human fibroblasts after 30 min treatment with increasing doses of recombinant hGH or serum (20% v/v) from participants obtained at rest or 15 min after resistance exercise. E–H, phosphorylation of the aforementioned proteins after 30 min treatment with increasing doses of recombinant IGF‐1. P‐values reflect differences between rest and exercise serum treatments. *Significantly different from control (P < 0.05). Values are in arbitrary units as means ± SD.
Statistics
There were no statistical differences between the pulsed and continuous style treatments; therefore, the data are presented as a single rest serum vs. exercise serum treatment comparison (RestTx and ExTx, respectively). Hormone concentrations, collagen content and tensile strength were analysed by Student's paired sample t‐test. Bioassays were analysed by one‐way ANOVA with Tukey's post hoc test for RestTx vs. ExTx (SigmaStat v.3.1, Systat Software, Point Richmond, CA, USA). Correlations and corresponding P‐value calculations were performed in GraphPad Prism (v.5, San Diego, CA, USA). Data are presented as means ± standard deviation.
Results
Exercise increases serum GH but not IGF‐1
Serum analysis by ELISA revealed that GH concentrations increased ∼7‐fold 15 min post‐exercise (P < 0.001 vs. rest; Fig. 1 A), whereas exercise did not increase serum IGF‐1 concentration at this time point (P = 0.21 vs. rest; Fig. 1 B).
Figure 1. Serum growth hormone (A) and insulin‐like growth factor 1 (B) concentrations at rest (open bars) and 15 min after acute resistance exercise (filled bars) .
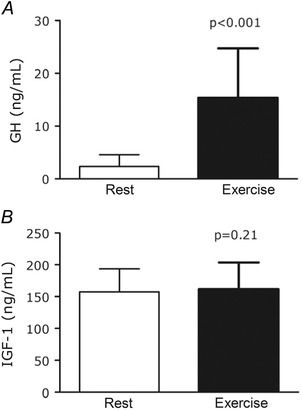
Serum was used to enrich media used to treat ligament constructs. P value from paired t‐test of Rest vs. Exercise; values are means ± SD.
Exercise serum increases collagen content and improves mechanical properties of engineered ligaments
Exercise serum treatment enhanced collagen content compared with rest serum treatment (215 ± 40 vs. 181 ± 33 μg collagen per construct, P = 0.001; Fig. 2 A), with serum from every individual showing a numerical increase in collagen within the treated grafts. The greater dry mass of ExTx constructs resulted in a similar percentage of collagen across conditions (∼7.5%; data not shown). ExTx also improved construct mechanical properties, significantly increasing maximal tensile load (+17%, P = 0.03 vs. RestTx; Fig. 2 B) and tending to increase ultimate tensile strength (+10%, P = 0.15 vs. RestTx; Fig. 2 C).
Figure 2. Collagen content (A), maximal tensile load (B), ultimate tensile strength (UTS) (C), and cross‐sectional area (CSA) (D) of ligament constructs treated for 7 days with medium that was enriched with serum obtained at rest (RestTx) and 15 min after acute resistance exercise (ExerciseTx) .
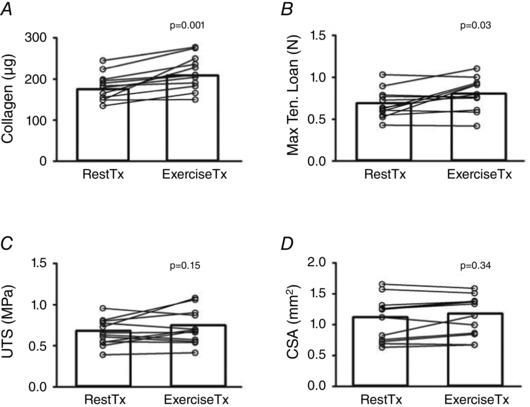
P value from paired t‐test of RestTx vs. ExerciseTx; values are means ± SD.
Exercise‐induced improvement of engineered ligament collagen content is not associated with increased GH
To examine potential associations of GH and IGF‐1 with collagen content in our ligament model, we correlated the change of each hormone in the serum with the change in ligament collagen. Surprisingly, the relative change in GH following exercise showed no relationship with the improvement in ligament collagen content (Fig. 3 A). In agreement with this finding, increasing concentrations of recombinant human GH (rhGH) had no effect on ligament collagen content or mechanical properties (Fig. 3 B–D).
In contrast to the GH effect, changes in IGF‐1 showed a trend towards a positive relationship with the improvement in collagen in the grafts (r 2 = 0.32, P = 0.071; Fig. 4 A). In support of this finding, increasing amounts of rhIGF‐1 resulted in increased collagen content (Fig. 4 B), and showed a strong trend toward improved MTL (P = 0.091; Fig. 4 C), and UTS (P = 0.051; Fig. 4 D). Importantly, however, once the level of IGF‐1 reached 150 ng ml−1 (Fig. 4 B), the level measured in the rest serum (Fig. 1 B), there was no further significant effect of IGF‐1 on ligament collagen content until 4 times the resting serum concentration.
Bioassays to determine molecular targets
To begin to understand which molecular targets were increased in the exercise serum and could be responsible for the improvement in collagen content, we performed bioassays comparing the molecular response of 2D cultured human fibroblasts to recombinant growth factors and the RestTx and ExTx serum. Nuclear abundance of phospho‐Smad2/3 increased in a dose‐dependent manner with rhTGF‐β1 treatment, but was not different between RestTx and ExTx serum (P = 0.28; Fig. 5). In contrast, phosphorylation of Akt, S6K1 and ERK1/2 was elevated after treatment with ExTx serum versus RestTx (Fig. 6 A, B and D). Treatment with rhGH had either a small (Akt and S6K1) or no (4EBP1 and ERK1/2) effect on the phosphorylation of these targets (Fig. 6 A–D). Treatment with rhIGF‐1 similarly had no effect on phosphorylation of 4E‐BP1 or ERK1/2 (Fig. 6 G and H). However, rhIGF‐1 treatment increased (P < 0.01) Akt and S6K1 phosphorylation at the lowest dose (75 ng ml−1). Similar to the effect on collagen, higher doses of IGF‐1 did not result in further increases (i.e. 150 and 300 ng ml−1) in Akt or S6K1 phosphorylation (Fig. 6 E and F).
Discussion
We used high intensity resistance exercise with short rest intervals to stimulate a large change in the biochemical milieu of human serum. We then used that serum to show that the enhanced biochemical milieu increased the collagen content of ligaments engineered from cells obtained from human ACL tissue. The increased collagen deposition that occurred with post‐exercise serum was functional since it enhanced engineered ligament tensile strength. Using a bioassay or direct measurement, none of the individual growth factors in the serum, identified a priori as pro‐collagen growth factors, readily explain the improved ligament function in the ExTx group. Instead, other activators of the PI3kinase/mTORC1 and ERK1/2 signalling pathways are likely to underlie the positive systemic effect of exercise on connective tissue.
This study aimed to combine the strengths of a physiologically derived intervention (a human exercise‐induced biochemical milieu) with the mechanistic advantages of an in vitro model within a highly controlled environment (the tissue engineered ligament). Thus while factors such as blood flow and hormone delivery (West et al. 2013) during the intervention were obviously lacking, we were able to examine the effects of a physiological biochemical milieu in serum per se on ligament tissue rather than artificially dosing with select recombinant hormones. Thus our data reflects the effects of the full complement of metabolites, hormones, isoforms, post‐translational modifications and binding proteins contained in serum at rest and after exercise.
We have previously illustrated (Schroeder et al. 2013) that the exercise‐induced testosterone response is of similar magnitude to variation that occurs during normal circadian rhythm. In contrast, peak GH concentrations after exercise (15–30 ng ml−1) are far greater than either basal growth hormone concentrations (0.2–4 ng ml−1; Sakharova et al. 2008) or even the concentrations resulting from exogenous GH administration (∼6 ng ml−1; Doessing et al. 2010 a). Further, exogenous delivery of GH in this range is known to be anabolic toward collagen (Doessing et al. 2010 a). Therefore, a rationale existed that an exercise‐induced biochemical milieu that was enriched in GH could increase collagen synthesis.
The effect of exercise serum that we observed on collagen content (+19%) is notable given that (i) the effect of pharmacological administration of GH on rates of collagen synthesis in tendon is relatively small (e.g. ∼1/5th of the effect compared to the effect on collagen in muscle; Doessing et al. 2010 a), and (ii) the treatment period was relatively brief (7 days). Surprisingly, rhGH alone had no effect on either the collagen content or the strength of the grafts. This is in contrast to the findings of Vestergaard and colleagues who showed that injection of rhGH into the patellar tendon increased the rate of collagen synthesis without any rise in circulating IGF‐1 (Vestergaard et al. 2012). The absence of an effect of rhGH on collagen content in the current study, together with the positive effect of IGF‐1 in our grafts, suggests that Vestergaard et al. were likely to be observing a local increase in IGF‐1 from either the patellar tendon tissue or surrounding tissues (e.g. through local GH‐induced production of IGF‐1). Olsen and colleagues have shown that IGF‐1 in the peritendinous fluid is ∼100‐fold lower than in the serum (Olesen et al. 2007). In this environment, our present work with rhIGF‐1 suggests that shifting from the very low IGF‐1 seen in the peritendinous space towards the level seen in the serum would dramatically stimulate collagen synthesis. However, in our study, IGF‐1 in the serum started at 150 ng ml−1, a level that showed near maximal collagen synthesis in our ligament model. Further, it is important to note that the ExTx had a beneficial effect on collagen even though average IGF‐1 levels were not changed, suggesting that altered IGF‐1 does not directly account for the increase in collagen synthesis induced by the ExTx.
If circulating IGF‐1 after exercise does not appear to drive the improvement in collagen synthesis then what did? TGF‐β family members are known to increase collagen synthesis. Since there are multiple isoforms of these proteins and exercise can increase circulating TGF‐β1 concentrations (Heinemeier et al. 2003), we performed a bioassay in 2D culture to measure TGF‐β1 activity. TGF‐β1 stimulates collagen gene expression in fibroblasts through the nuclear translocation of Smad 2/3 (Massague, 1998; Chen et al. 1999). Using the nuclear abundance of phosphorylated Smad2/3 as a marker of activation by TGF‐β1, we demonstrated that increasing recombinant TGF‐β1 increases nuclear Smad2/3 in a dose‐dependent manner in human ACL fibroblasts. However, the amount of nuclear Smad2/3 induced by resting human serum was already equivalent to that induced by the amount of TGF‐β1 used to stimulate collagen in our model (Hagerty et al. 2012). Further, there was no enhancement of nuclear Smad2/3 by the exercise serum, indicating that TGF‐β1 activity does not explain the positive effects of the ExTx on collagen production.
The bioassays with the rest and exercise serum did show that both the PI3K/mTORC1 and ERK1/2 pathways were more activated in the presence of the ExTx. mTORC1, which is downstream of PI3K and Akt in canonical growth factor signalling, propagates cellular growth signals via S6K1 and 4E‐BP1 (Sengupta et al. 2010) and positively regulates collagen production (Shegogue & Trojanowska, 2004). Exercise serum enhanced the phosphorylation of the Akt and S6K1, suggestive of canonical activation of mTORC1 by growth factors (Fig. 6). Recombinant hGH and hIGF‐1 treatment had a small (Akt and S6K1) or no (4EBP1) effect on PI3K/mTORC1 signalling with both factors showing the maximal effect at the lowest dose. Thus, it is important to note that the recombinant hIGF‐1 dose at which S6K phosphorylation plateaued was below the physiological concentrations (150 ng ml−1) measured in the serum of our participants. This suggests that basal circulating IGF‐1 may be permissive but is not likely to be stimulatory for S6K phosphorylation and, potentially, collagen synthesis in the ExTx group. Taken together, the PI3K/mTORC1 pathway is stimulated in fibroblasts by the post‐exercise hormonal milieu, and these effects are not reproduced by the addition of individual growth factors (neither GH, nor IGF‐1, nor TGF‐β1).
ERK1/2 signalling has previously been shown to be essential to load‐induced collagen synthesis (Papakrivopoulou et al. 2004). Previous work by our lab in vitro demonstrated that acute ERK1/2 phosphorylation predicted collagen synthesis in response to mechanical stretch (Paxton et al. 2012). In the present study, acute treatment with post‐exercise serum increased ERK1/2 phosphorylation compared with treatment with rest serum; this increase appeared to be independent of hGH or hIGF‐1 since neither recombinant hGH nor hIGF‐1 increased phosphorylation of ERK1/2 at the doses tested. Taken together, the ERK pathway appears to be responsive to both mechanical stretch (Paxton et al. 2012), as well as the post‐exercise biochemical milieu (present data). Further work is needed to determine whether either or both PI3K/mTORC1 and/or ERK1/2 are required for the positive effects of the ExTx on engineered ligament collagen content and mechanics.
As with any in vitro experiment, the advantage of a more controlled environment is balanced with the limitations in the model. It is possible that ACL cells from different tissue donors could have produced different responses from the ones that were observed (Lo et al. 1998). However, we have previously shown that the variability between ACL donors using this model is low (Lee et al. 2015). Another potential limitation is the isolation of cells from ACL tissue that was surgically removed following injury. However, while cells from injured or intact ACL can exhibit differences in phenotype, Brune et al. (2007) have shown these two populations exhibit similar cell morphology, tissue organization, and expression of integrins, elastin and smooth muscle actin.
In summary, we report that ligament constructs treated for 7 days with serum obtained post‐exercise exhibit enhanced collagen content and tensile strength compared with constructs treated with serum obtained from the same individuals at rest. Thus, our data suggest that one biological function of the exercise‐induced biochemical milieu may be to support connective tissue remodelling. Mechanistically, bioassays of fibroblasts in 2D culture suggest that the enhanced phenotype may be mediated by the activation of the PI3K/mTORC1 and ERK1/2 pathways, both of which were increased by the post‐exercise serum. From an applied standpoint, our findings suggest that changes in the physiological milieu may provide a signal to stimulate collagen turnover in tendon, ligaments and skin (Crane et al. 2015) to maintain or improve tissue function.
Additional information
Competing interests
The authors report no competing interests.
Author contributions
Conception and design of the experiments: D.W.D.W., A.L., T.M., B.S., K.B. Collection, analysis and interpretation of data: D.W.D.W., A.L., T.M., B.S., C.L., K.B. Drafting the article or revising it critically for important intellectual content: D.W.D.W., A.L., K.B. All authors read and approved the final version of the manuscript and all authors listed qualify for authorship.
Funding
D.W.D.W. was supported by postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada; A.L. was supported by an ARCS Foundation Scholarship. This work was supported by a UC Davis College of Biological Sciences grant to K.B.
Acknowledgements
We thank our exercising participants for their time and effort. We are grateful to the ACL donor for the tissue used in this study.
References
- Baehr LM, Tunzi M & Bodine SC (2014). Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton‐Davis ER, Shoturma DI, Musaro A, Rosenthal N & Sweeney HL (1998). Viral mediated expression of insulin‐like growth factor I blocks the aging‐related loss of skeletal muscle function. Proc Natl Acad Sci U S A 95, 15603–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer ML, Yeung CY, Kadler KE, Qvortrup K, Baar K, Svensson RB, Magnusson SP, Krogsgaard M, Koch M & Kjaer M (2010). The initiation of embryonic‐like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials 31, 4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesen AP, Dideriksen K, Couppe C, Magnusson SP, Schjerling P, Boesen M, Aagaard P, Kjaer M & Langberg H (2014). Effect of growth hormone on aging connective tissue in muscle and tendon: gene expression, morphology, and function following immobilization and rehabilitation. J Appl Physiol 116, 192–203. [DOI] [PubMed] [Google Scholar]
- Brune T, Borel A, Gilbert TW, Fraceschi JP, Badylak SF & Sommer P (2007). In vitro comparison of human fibroblasts from intact and ruptured ACL for use in tissue engineering. Eur Cells Mater 14, 78–90. [DOI] [PubMed] [Google Scholar]
- Calve S, Lytle IF, Grosh K, Brown DL & Arruda EM (2010). Implantation increases tensile strength and collagen content of self‐assembled tendon constructs. J Appl Physiol 108, 875–881. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M & Varga J (1999). Stimulation of type I collagen transcription in human skin fibroblasts by TGF‐β: involvement of Smad 3. J Invest Dermatol 112, 49–57. [DOI] [PubMed] [Google Scholar]
- Crane JD, MacNeil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, Kemp BE, Raha S, Steinberg GR & Tarnopolsky MA (2015). Exercise‐stimulated interleukin‐15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 14, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers LB, Jansen DC, van Veen‐Reurings A, van den Bos T & Everts V (1997). Microassay for the assessment of low levels of hydroxyproline. Biotechniques 22, 656–658. [DOI] [PubMed] [Google Scholar]
- Dodt C, Breckling U, Derad I, Fehm HL & Born J (1997). Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension 30, 71–76. [DOI] [PubMed] [Google Scholar]
- Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen MH, Flyvbjerg A & Kjaer M (2010. a). Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol 588, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doessing S, Holm L, Heinemeier KM, Feldt‐Rasmussen U, Schjerling P, Qvortrup K, Larsen JO, Nielsen RH, Flyvbjerg A & Kjaer M (2010. b). GH and IGF1 levels are positively associated with musculotendinous collagen expression: experiments in acromegalic and GH deficiency patients. Eur J Endocrinol 163, 853–862. [DOI] [PubMed] [Google Scholar]
- Doessing S & Kjaer M (2005). Growth hormone and connective tissue in exercise. Scand J Med Sci Sports 15, 202–210. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Lambert DL, Starkie RL, Proietto J & Hargreaves M (1998). Effect of epinephrine on muscle glycogenolysis during exercise in trained men. J Appl Physiol 84, 465–470. [DOI] [PubMed] [Google Scholar]
- Hagerty P, Lee A, Calve S, Lee CA, Vidal M & Baar K (2012). The effect of growth factors on both collagen synthesis and tensile strength of engineered human ligaments. Biomaterials 33, 6355–6361. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H & Kjaer M (2003). Exercise‐induced changes in circulating levels of transforming growth factor‐β‐1 in humans: methodological considerations. Eur J Appl Physiol 90, 171–177. [DOI] [PubMed] [Google Scholar]
- Jones JI & Clemmons DR (1995). Insulin‐like growth factors and their binding proteins: biological actions. Endocr Rev 16, 3–34. [DOI] [PubMed] [Google Scholar]
- Kapacee Z, Richardson SH, Lu Y, Starborg T, Holmes DF, Baar K & Kadler KE (2008). Tension is required for fibripositor formation. Matrix Biol 27, 371–375. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Secher NH, Bach FW & Galbo H (1987). Role of motor center activity for hormonal changes and substrate mobilization in humans. Am J Physiol Regul Integr Comp Physiol 253, R687–695. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Aguilera BA, Terada M, Newton RU, Lynch JM, Rosendaal G, McBride JM, Gordon SE & Hakkinen K (1995). Responses of IGF‐I to endogenous increases in growth hormone after heavy‐resistance exercise. J Appl Physiol 79, 1310–1315. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Dunn‐Lewis C, Comstock BA, Thomas GA, Clark JE & Nindl BC (2010). Growth hormone, exercise, and athletic performance: a continued evolution of complexity. Curr Sports Med Rep 9, 242–252. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Gordon SE, Fleck SJ, Marchitelli LJ, Mello R, Dziados JE, Friedl K, Harman E, Maresh C & Fry AC (1991). Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med 12, 228–235. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, Frykman P, McCurry D & Fleck SJ (1990). Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol 69, 1442–1450. [DOI] [PubMed] [Google Scholar]
- Lee CA, Lee‐Barthel A, Marquino L, Sandoval N, Marcotte GR & Baar K (2015). Estrogen inhibits lysyl oxidase and decreases mechanical function in engineered ligaments. J Appl Physiol 118, 1250–1257. [DOI] [PubMed] [Google Scholar]
- Lo IKY, Marchuk LL, Hart DA & Frank CB (1998). Comparison of mRNA levels for matrix molecules in normal and disrupted human anterior cruciate ligaments using reverse transcription‐polymerase chain reaction. J Orthop Res 16, 421–428. [DOI] [PubMed] [Google Scholar]
- Louard RJ, Bhushan R, Gelfand RA, Barrett EJ & Sherwin RS (1994). Glucocorticoids antagonize insulin's antiproteolytic action on skeletal muscle in humans. J Clin Endocrinol Metab 79, 278–284. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Heinemeier KM, Koskinen SO & Kjaer M (2008). Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connect Tissue Res 49, 165–168. [DOI] [PubMed] [Google Scholar]
- Marturano JE, Xylas JF, Sridharan GV, Georgakoudi I & Kuo CK (2014). Lysyl oxidase‐mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation. Acta Biomater 10, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J (1998). TGF‐β signal transduction. Annu Rev Biochem 67, 753–791. [DOI] [PubMed] [Google Scholar]
- Moller N, Schmitz O, Porksen N, Moller J & Jorgensen JO (1992). Dose‐response studies on the metabolic effects of a growth hormone pulse in humans. Metabolism 41, 172–175. [DOI] [PubMed] [Google Scholar]
- Nindl BC, Urso ML, Pierce JR, Scofield DE, Barnes BR, Kraemer WJ, Anderson JM, Maresh CM, Beasley KN & Zambraski EJ (2012). IGF‐I measurement across blood, interstitial fluid, and muscle biocompartments following explosive, high‐power exercise. Am J Physiol Regul Integr Comp Physiol 303, R1080–R1089. [DOI] [PubMed] [Google Scholar]
- Olesen JL, Heinemeier KM, Gemmer C, Kjaer M, Flyvbjerg A & Langberg H (2007). Exercise‐dependent IGF‐I, IGFBPs, and type I collagen changes in human peritendinous connective tissue determined by microdialysis. J Appl Physiol 102, 214–220. [DOI] [PubMed] [Google Scholar]
- Papakrivopoulou J, Lindahl GE, Bishop JE & Laurent GJ (2004). Differential roles of extracellular signal‐regulated kinase 1/2 and p38MAPK in mechanical load‐induced procollagen α1(I) gene expression in cardiac fibroblasts. Cardiovasc Res 61, 736–744. [DOI] [PubMed] [Google Scholar]
- Paxton JZ, Donnelly K, Keatch RP & Baar K (2009). Engineering the bone‐ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng Part A 15, 1201–1209. [DOI] [PubMed] [Google Scholar]
- Paxton JZ, Donnelly K, Keatch RP, Baar K & Grover LM (2010. a). Factors affecting the longevity and strength in an in vitro model of the bone‐ligament interface. Ann Biomed Eng 38, 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JZ, Grover LM & Baar K (2010. b). Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A 16, 3515–3525. [DOI] [PubMed] [Google Scholar]
- Paxton JZ, Hagerty P, Andrick JJ & Baar K (2012). Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A 18, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine L, Hauger RL, Gillin JC & Irwin M (2000). Effects of sleep and sleep deprivation on interleukin‐6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab 85, 3597–3603. [DOI] [PubMed] [Google Scholar]
- Sakharova AA, Horowitz JF, Surya S, Goldenberg N, Harber MP, Symons K & Barkan A (2008). Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J Clin Endocrinol Metab 93, 2755–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder ET, Villanueva M, West DD & Phillips SM (2013). Are acute post‐resistance exercise increases in testosterone, growth hormone, and IGF‐1 necessary to stimulate skeletal muscle anabolism and hypertrophy? Med Sci Sports Exerc 45, 2044–2051. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR & Sabatini DM (2010). Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40, 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shegogue D & Trojanowska M (2004). Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3‐kinase‐independent pathway. J Biol Chem 279, 23166–23175. [DOI] [PubMed] [Google Scholar]
- Spriet LL, Ren JM & Hultman E (1988). Epinephrine infusion enhances muscle glycogenolysis during prolonged electrical stimulation. J Appl Physiol 64, 1439–1444. [DOI] [PubMed] [Google Scholar]
- Sutton J & Lazarus L (1976). Growth hormone in exercise: comparison of physiological and pharmacological stimuli. J Appl Physiol 41, 523–527. [DOI] [PubMed] [Google Scholar]
- Vahl N, Moller N, Lauritzen T, Christiansen JS & Jorgensen JO (1997). Metabolic effects and pharmacokinetics of a growth hormone pulse in healthy adults: relation to age, sex, and body composition. J Clin Endocrinol Metab 82, 3612–3618. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Jorgensen JO, Olesen JL, Bosnjak E, Holm L, Frystyk J, Langberg H, Kjaer M & Hansen M (2012). Local administration of growth hormone stimulates tendon collagen synthesis in elderly men. J Appl Physiol 113, 1432–1438. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Zimmerman JC, Czeisler CA & Ronda J (1983). Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab 56, 352–358. [DOI] [PubMed] [Google Scholar]
- West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK & Phillips SM (2010). Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training‐induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol 108, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Cotie LM, Mitchell CJ, Churchward‐Venne TA, MacDonald MJ & Phillips SM (2013). Resistance exercise order does not determine postexercise delivery of testosterone, growth hormone, and IGF‐1 to skeletal muscle. Appl Physiol Nutr Metab 38, 220–226. [DOI] [PubMed] [Google Scholar]
- West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK & Phillips SM (2009). Resistance exercise‐induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587, 5239–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, Grant EJ, Correia CE & Phillips SM (2006). Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol 98, 546–555. [DOI] [PubMed] [Google Scholar]


