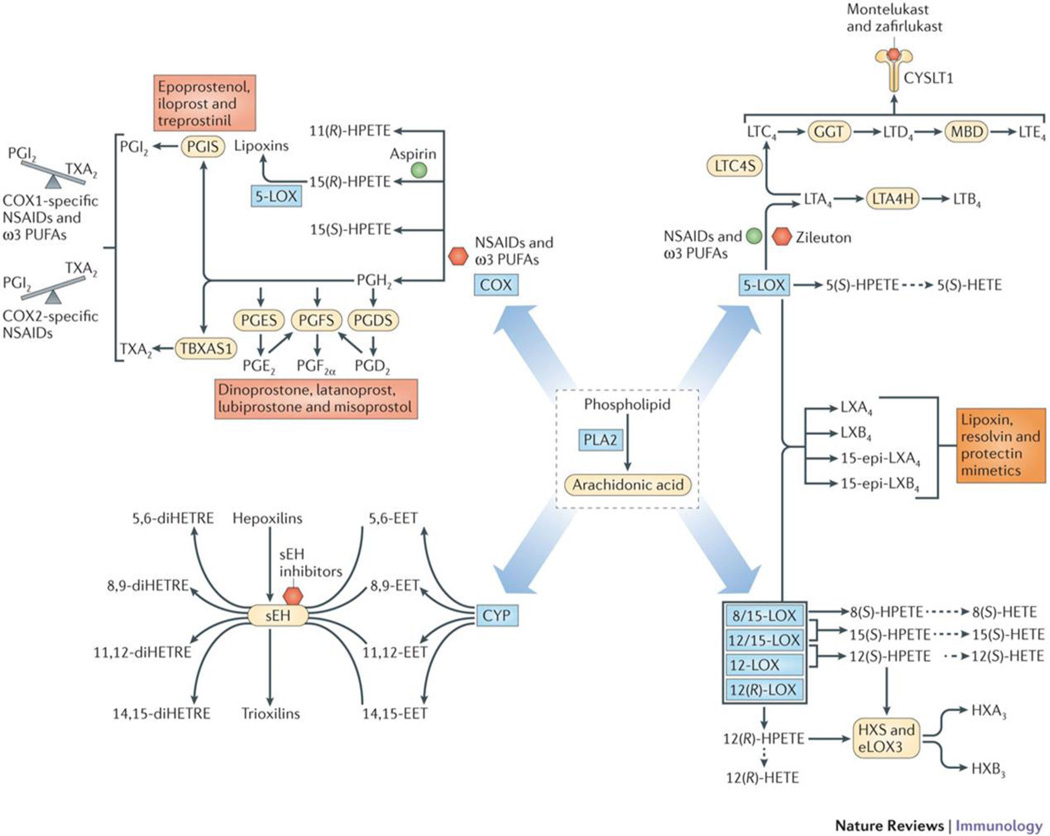
Figure 4. Therapeutics targeting eicosanoid pathways.
Enzymes in the cyclooxygenase pathway generate prostaglandins, thromboxanes, and lipoxins; lipoxygenase pathway enzymes generate leukotrienes, HETEs, and hepoxilins; and cytochrome P450 epoxyhydrolase pathway enzymes generate epoxides and dihydroxy polyunsaturated fatty acids (PUFA). All of the pathways have pharmacological intervention points including enzyme inhibitors and receptor antagonists (red hexagon), product enhancers (green circle) and mimetics (red box). NSAIDs and ω3 PUFAs inhibit COX formation of each product derived from AA (red hexagon), with the exception of one NSAID, aspirin, which enhances COX-2 formation of 15(R)-HETE (green circle) that can be converted to lipoxins by 5-LOX. COX-1-specific NSAIDS and ω3 PUFAs shift the vascular balance to higher PGI2, whereas COX-2 specific NSAIDs shift the vascular balance to higher TxA2 (gray), which are in opposite directions due to coupling of platelet COX-1 with TXAS and endothelial COX-2 with PGIS; analogs of prostaglandins are used clinically to mimic endogenous bioactivity (red box). Montelukast and zafirlukast specifically inhibit activation of cysLT1 (red hexagon). Zileuton inhibits 5-LOX conversion of arachidonic acid (red hexagon), but NSAIDs and ω3 PUFAs can increase conversion (green circle) via shunting of AA from inhibited COX-1 and COX-2. Inhibitors of sEH reduce inactivation of EETs as well as hepoxilins (red hexagon). Lipoxin, resolvin, and protectin mimetics (orange box) are being developed for treatment of ocular, periodontal, and cardiovascular diseases.