Fig. 3.
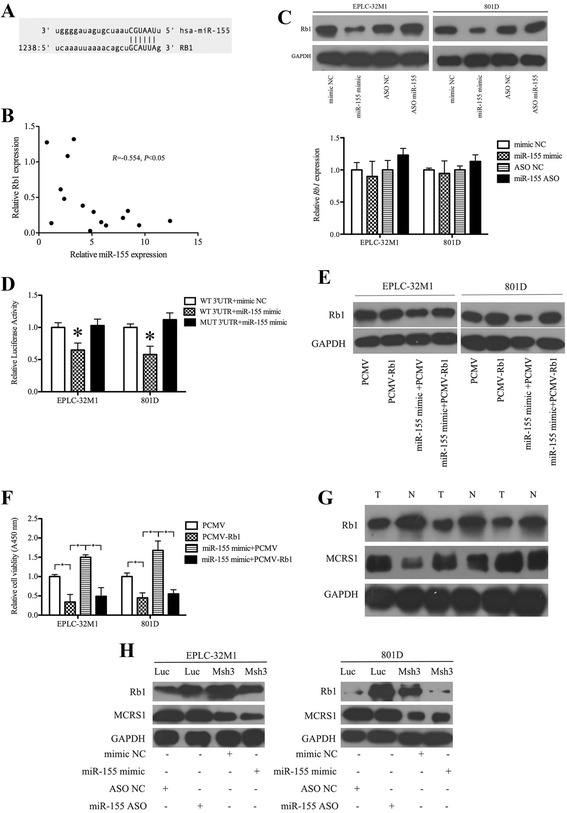
MCRS1 regulated cellular proliferation via miR-155 targeting Rb1 mRNA in NSCLCs. a Schematic of the putative miR-155 binding site in the Rb1 3′UTR region. b The results of the correlation analysis of the levels of miR-155 and Rb1 protein in NSCLC tissues (Pearson’s method, R =−0.554, *P < 0.05). c Relative levels of expression of Rb1 protein (top) and Rb1 mRNA (bottom) in cells transfected with the miR-155 mimic, the NC mimic, an miR-155 inhibitor (miR-155 ASO) or NC ASO, respectively. (Student’s t-test, *P < 0.05). d Validation of the direct targeting of Rb1 mRNA by miR-155 was obtained using a luciferase reporter assay. WT: wild-type Rb1 3′UTR; MUT: mutant Rb1 3′UTR. (Student’s t-test, *P < 0.05). e The inhibition of Rb1 expression by miR-155 was partially reversed by co-transfection with pCMV-Rb1 (to drive the ectopic expression of Rb1 mRNA lacking the 3′UTR to which miR-155 bound) and the miR-155 mimic. f The level of cell viability was reduced by transfection with PCMV-Rb1. Importantly, the induction of a higher level of cell viability by the miR-155 mimic was partially prevented by PCMV-Rb1 transfection. g The relative levels of expression of Rb1 and MCRS1 protein in clinical samples. T: Tumor; N: Noncancerous. h The lower level of Rb1 protein in cancer cells without MCRS1 silencing (Luc) was increased by treatment with the miR-155 inhibitor, and the higher level of Rb1 protein in the MCRS1-knockdown cells (Msh3) was decreased by treatment with the miR-155 mimic. NC: negative control. ASO: antisense oligonucleotide
