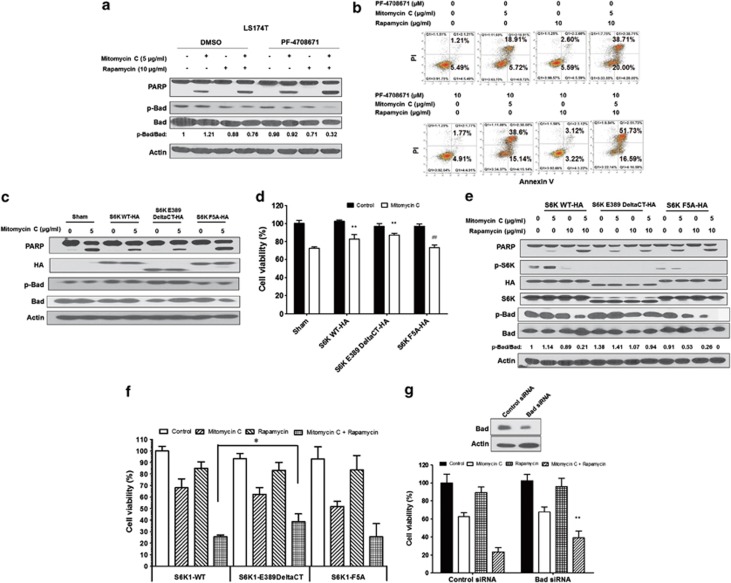
Figure 5.
Role of S6K1 in the combination of mitomycin C and rapamycin-induced apoptosis. (a) LS174T cells were pretreated with 10 μM PF-4708671 for 30 min followed by mitomycin C (5 μg/ml) and/or rapamycin (10 μg/ml) for 24 h. PARP cleavage and p-Bad/Bad were detected by immunoblotting. Actin was used to confirm the equal amount of proteins loaded in each lane. (b) After treatment, cells were stained with Annexin V and PI. Apoptosis was detected by the flow cytometric assay. (c) LS174T cells were transiently transfected with a sham plasmid, S6K1 WT-HA, S6K1 E389 DeltaCT-HA and S6K1 F5A-HA; 48 h later, cells were treated with mitomycin C (5 μg/ml) for 24 h. PARP, HA, and p-Bad/Bad were detected by immunoblotting. Actin was shown as an internal control. (d) Cell viability was analyzed by MTS assay. **P<0.01 compared with the sham group; ##P<0.01 compared with the S6K WT group. (e) LS174T cells were transiently transfected with S6K1 WT-HA, S6K1 E389 DeltaCT-HA and S6K1 F5A-HA; 48 h later, cells were treated with mitomycin C (5 μg/ml) and/or rapamycin (10 μg/ml) for 24 h. PARP, HA, p-S6K1/S6K1 and p-Bad/Bad were detected by immunoblotting. Actin was shown as an internal standard. (f) LS174T cells were transiently transfected with S6K1 WT-HA, S6K1 E389 DeltaCT-HA and S6K1 F5A-HA; 48 h later, cells were treated with mitomycin C (5 μg/ml) and/or rapamycin (10 μg/ml) for 24 h. Cell viability was analyzed by MTS assay. Error bars represent S.D. from triplicate experiments. The asterisk (*) represents a statistically significant difference between the two groups (P<0.05). (g) LS174T cells were transfected with control siRNA or Bad siRNA. After 48 h, cells were treated with mitomycin C (5 μg/ml) and/or rapamycin (10 μg/ml). Cell viability was analyzed by MTS assay 24 h later. The levels of Bad were detected by immunoblotting 72 h after transfection. Actin was used as a loading control. The asterisk (**) represents a statistically significant difference compared with the control siRNA group (P<0.01)