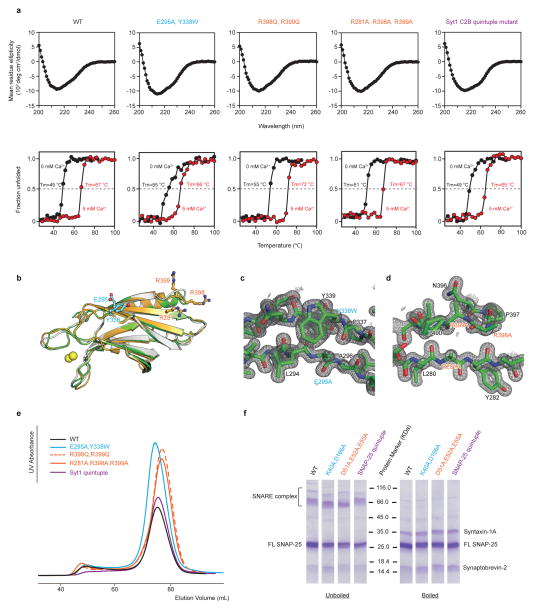
Extended Data Figure 7. Syt1 and SNAP-25 mutants are well folded.
a, Upper panels, CD spectra of WT and mutant Syt1 C2B domains in the absence of Ca2+. Lower panels, thermal denaturation was monitored by molar ellipticity at a wavelength of 216 nm in the absence of Ca2+ (black) and in the presence of 5 mM Ca2+ (red). The specified melting temperatures were estimated as the mid-point of the melting curves (Methods). b, Superposition of the Syt1 C2B domains from the Ca2+-bound Syt1-SNARE complex in the short unit cell crystal form (gold), the crystal structure of the quintuple mutant (R281A, E295A, Y338W, R398A, R399A) of the Syt1 C2B domain (green), and the crystal structure of the isolated Syt1 C2B domain (white, PDB code 2YOA). c and d, Representative m2Fo-DFc electron density maps of the crystal structure of the quintuple mutant of the Syt1 C2B domain (Extended Data Table 1) contoured at 2.0 σ. The labels refer to the mutated residues. e, Overlay of SEC profiles of full-length Syt1 mutant proteins used in the single vesicle-vesicle fusion assay (Figs. 3d–g). f, Coomassie blue-stained SDS-PAGE with and without boiling of neuronal SNARE complexes formed by full-length SNAP-25 and its mutants, syntaxin-1A, and synaptobrevin-2, using the proteins that were used in the single vesicle-vesicle fusion assay (Methods).