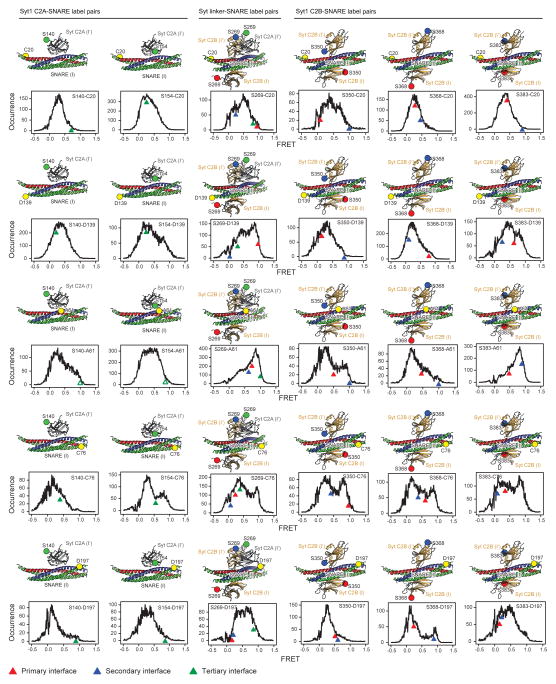
Extended Data Figure 4. Single molecule FRET efficiency distributions of the Syt1-SNARE complex vs. FRET efficiency values calculated from the Syt1-SNARE interfaces observed in the crystal structure.
Shown are histograms of intermolecular single molecule FRET (smFRET) efficiency values that were measured between pairs of covalently attached organic labels on the Syt1 C2AB fragment and the SNARE complex28 (also shown as large spheres superimposed on the interfaces observed in the crystal structure). Arrowheads indicate FRET efficiencies calculated from the crystal structure of the Ca2+-bound Syt1-SNARE complex in the long unit cell crystal form (complex I) for the primary, secondary and tertiary interfaces, using the methods and approximations described in ref. 28 to simulate the positions of dye centers in order to calculate the FRET-efficiency values. Only the dye pair combinations between the nearest C2 domain (including the C2A-C2B linker) and the SNARE complex were calculated for the three interfaces. Note that due to the presence of transitions between different states the histogram reflect a combined effect of interaction interfaces. The label at position A61 would have disrupted the tertiary interfaces between the C2A domain and the SNARE complex, explaining the discrepancy for these labels (indicated by open triangles). In retrospect, the top smFRET-derived model28 and the primary interface observed in the crystal structure primarily differed in the orientation of the C2B domain. Moreover, the top smFRET derived model predicted the approximate location primary interface on the neuronal SNARE (see Fig. 4c in ref. 28).