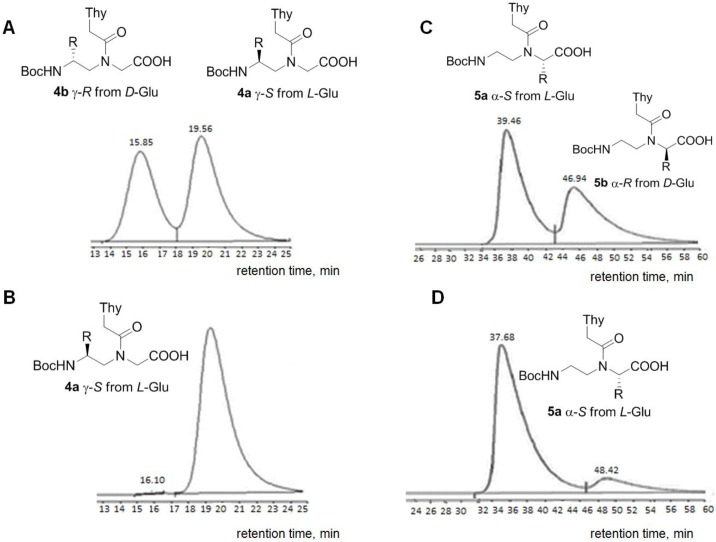
Fig 2. Chromatographic profiles of the PNA monomers (enantiomers).
(A) Mixture of 4a and 4b (S- and R- enantiomers of the γ-monomer); (B) S-entantiomer of the γ-monomer, 4a (Conditions: Diasphere column 110-Chirasel-E-PA, 7 μm; elution system: MeOH/CH3COOH = 96/4 (v/v); flow rate: 1 mL/min; UV-detection at 254 nm; temperature: 20°C); (C) Mixture of 5a and 5b (R- and S-enantiomers of the α-monomer); (D) S-entantiomer of the α-monomer, 5a (Conditions: Diasphere column 110-Chirasel-E-PA, 7 μm; elution system: MeOH/CH3COOH/TEA = 100/0.1/0.1 (v/v/v); flow rate: 0.5 mL/min; UV-detection at 254 nm; temperature: 20°C); R = CH2CH2COOBn.