Figure 1.
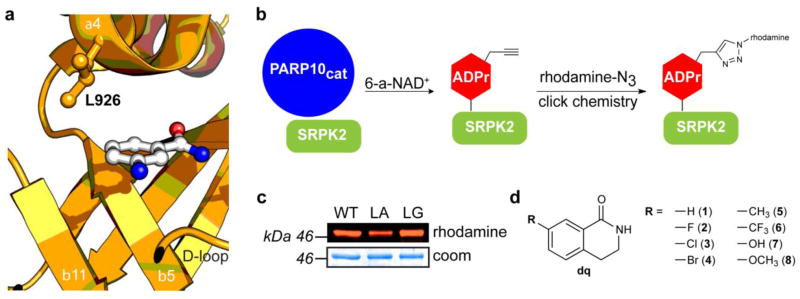
A chemical genetics strategy for generating selective inhibitors of PARP10. (a) Structure of PARP10 with nicotinamide isostere 3-aminobenzamide (3-AB) (PDB ID: 3HKV) with mutated residue (L926) indicated. (b) Schematic showing the PARP10-mediated transfer of alkyne-tagged ADP-ribose onto SRPK2 from 6-a-NAD+, followed by click conjugation with a fluorescent azide reporter (rhodamine-N3). (c) Activity comparison of engineered PARP10cat mutants L926A (LA) and L926G (LG) to WT-PARP10cat. (d) Structure of C-7 substituted dq analogues 1 – 8 designed to selectively inhibit engineered PARP10 mutants.
