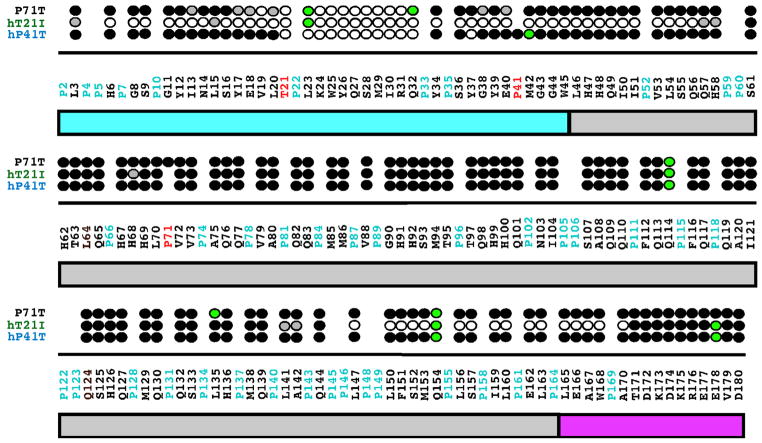
Fig. 6.
Summary of the amide resonances that partially disappear (grey-filled circles) or completely disappear (open circles) in the 1H–15N HSQC spectra of 15N-labeled hP41T, hT21I, and P71T when the concentration of the protein is increased from 0.1 to 1.8 mM. Amide cross peaks whose intensity change little over the concentration range are indicated by solid circles and cross peaks that could not be tracked unambiguously are indicated by green-filled circles. The full murine amelogenin sequence is shown with the proline residues highlighted in cyan and the site of the individual point mutations in each protein highlighted in red. Underneath the primary sequence is a schematic illustration of the three major regions of the protein: TRAP region (cyan); HQP-rich region (grey); and hydrophilic C-terminal region (magenta). The data for hP41T and hT21I were published previously [31]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)