Abstract
Although the essential element manganese (Mn) is neurotoxic at high doses, the effects of lower exposure are unclear. MRI T1-weighted (TIW) imaging has been used to estimate brain Mn exposure via the pallidal index (PI), defined as the T1W intensity ratio in the globus pallidus (GP) versus frontal white matter (FWM). PI may not, however, be sensitive to Mn in GP because Mn also may accumulate in FWM. This study explored: (1) whether T1 relaxation rate (R1) could quantify brain Mn accumulation more sensitively; and (2) the dose-response relationship between estimated Mn exposure and T1 relaxation rate (R1). Thirty-five active welders and 30 controls were studied. Occupational questionnaires were used to estimate hours welding in the past 90 days (HrsW) and lifetime measures of Mn exposure. T1W imaging and T1-measurement were utilized to generate PI and R1 values in brain regions of interest (ROIs). PI did not show a significant association with any measure of Mn and/or welding-related exposure. Conversely, in several ROIs, R1 showed a nonlinear relationship to HrsW, with R1 signal increasing only after a critical exposure was reached. The GP had the greatest rate of Mn accumulation. Welders with higher exposure showed significantly higher R1 compared either with controls or with welders with lower exposure. Our data are additional evidence that Mn accumulation can be assessed more sensitively by R1 than by PI. Moreover, the nonlinear relationship between welding exposure and Mn brain accumulation should be considered in future studies and policies.
Keywords: welders, manganese, MRI, R1, pallidal index
Manganese (Mn), although an essential nutrient, can be toxic at high doses, causing neurological effects such as parkinsonism and dystonia (Cersosimo and Koller, 2006; Racette et al., 2005), as well as cognitive and behavioral deficits (Bowler et al., 2006; Dobson et al., 2004; Flynn and Susi, 2009). There is, however, uncertainty regarding the occupational and public health consequences of Mn exposure, especially when the exposure level is relatively low. This is due partly to the lack of an objective in vivo marker of Mn concentration in human brain, and partly because of insufficient data on how dose correlates with exposure. In addition, the toxicokinetics of Mn accumulation in brain are complex and not well understood.
It is known that the major route for transport of inhaled metals into the brain is via the blood-brain-barrier (BBB). Mn absorption from the gut is ∼3%, whereas lung absorption is often assumed to be complete (Williams et al., 2012), although Mn solubility is influenced by a variety of factors including the presence of potassium, fluoride and the fume particle size distribution (Taube, 2013). A recent analysis suggests that diet is the predominant source of Mn in the blood when air concentrations are <0.010 mg/m3 (Baker et al., 2014b). Efflux of Mn from the brain is by slow diffusion (Yokel, 2009), and the average half-life in brain has been estimated at over 50 days (Newland et al., 1987), leading to potential accumulation.
Mn has paramagnetic characteristics and can shorten the MRI longitudinal relaxation time (T1) and increase T1-weighted intensity (T1WI). Indeed, Mn has the highest T1 relaxivity (6.67/mM/s) among all metals, higher even than the most commonly used clinical contrast T1 agent gadolinium (5.0/mM/s). Other paramagnetic metals in welding fumes have significantly less T1 relaxivity (Cr3+ = 3.13; Ni2+ = 0.5; Cu2+ = 0.5, Fe2+ = 0.01) (Gallez et al., 2001; Yilmaz et al., 1999). For this reason, T1WI imaging has been used to assess Mn accumulation in brain tissue (Baker et al., 2014a; Pal et al., 1998; Sen et al., 2011) by means of the pallidal index (PI), the ratio of T1WI in the GP [where Mn appears to accumulate most significantly (Dorman et al., 2006)] compared with intensity in the orbitofrontal white matter (OFWM). Previous studies, however, suggested that Mn also can enter the OFWM as well as other brain regions. Theoretically, this would make the PI less accurate than R1 (1/T1) in assessing the concentration of Mn in the brain (Choi et al., 2007; Dorman et al., 2006).
Older MRI sequences, however, required long acquisition times to obtain T1, decreasing their utility for application to human studies (Kesselring et al., 1990). Studies assessing T1 in human brain, therefore, were sparse, except for one attempt to measure R1 in a focused region (olfactory bulb; Sen et al., 2011) and another that measured T1 only in the GP (Choi et al., 2007). The recent development of fast T1 mapping imaging techniques enabled T1 images to be acquired in a much shorter time (Venkatesan et al., 1998), and this study utilized this technological advance to measure R1 and gauge tissue-specific Mn accumulation in several regions of interest (ROIs) beyond the GP.
The primary objective of the study was to examine R1 as a biomarker of Mn accumulation in the brain by assessing associations with short- and long-term welding exposure measures. We account for potential confounders and coindicators of welding fume exposure such as blood metal levels [eg, Cr, Cu, Fe, K, and Pb]. Our goal was to test the following hypotheses: (1) blood levels of these metals would be higher in welders than in controls; (2) both PI and R1 would be greater in brain ROIs in welders than in controls; and (3) R1 would provide a more sensitive exposure measure than PI. In addition, we also explored the dose-response relationship between estimated Mn exposure and T1 relaxation rate (R1).
MATERIALS AND METHODS
Subjects
Forty-one welders and 40 controls were recruited initially from the meetings of regional unions in Philadelphia and Harrisburg, Pennsylvania, and from the community around the Penn State Milton S. Hershey Medical Center. A detailed questionnaire confirmed that 6 welders had not welded over the preceding 90 days, and 10 controls had a history of welding sometime during their life. Thus, the final analysis includes 35 welders (45.8 ± 11.2 years old) with recent exposure (within 90 days) and 30 controls (43.5 ± 11.6 years old) with no history of welding (see demographic information in Table 1-I). All subjects were male and answered negatively for past diagnosis of Parkinson’s disease (PD). Welders represented several different trades and industry groups (e.g., boilermakers, pipefitters, pile drivers, railroad welders, and a variety of different manufacturing jobs). Controls were age-matched volunteers from the community of the same region with various occupations with no history of welding.
TABLE 1.
Summary Statistics for Demographics and Exposure Metrics (I); Blood Metals (II); and MRI Measures—R1 Regional Values and PI (III) in Welders and Controls
| Welders (N = 35) | Controls (N = 30) | P-Values* | |
|---|---|---|---|
| Mean ± SD (Median) | Mean ± SD (Median) | ||
| I. Demographics and exposure metrics | |||
| Age (years) | 45.9 ± 11.2 (51) | 43.6 ± 11.5 (42.5) | 0.51 |
| Education (years) | 12.8 ± 1.6 (12) | 16.2 ± 2.4 (16) | <10−6 |
| HrsW (h) | 309 ± 172 (360) | 0 ± 0 (0) | <10−12 |
| YrsW (years) | 25.3 ± 11.2 (29) | 0 ± 0 (0) | <10−12 |
| E90 (mg/m3—days) | 3.0 ± 1.7 (2.4) | 0.0027 ± 0 (0.0027) | <10−12 |
| ELT (mg/m3—years) | 1.2 ± 0.8 (1.1) | 0.0013 ± 0.0003 (0.0013) | <10−12 |
| AST (IU/l) | 36.5 ± 8.7 (36) | 35.7 ± 9.4 (35) | 0.65 |
| ALT (IU/l) | 39.7 ± 18.2 (37) | 38.1 ± 16.9 (31) | 0.56 |
| BMI | 28.8 ± 5.8 (27.9) | 25.3 ± 5.3 (26.3) | 0.031 |
| Hemoglobin | 15.4 ± 0.9 (15.5) | 14.8 ± 0.8 (14.9) | 0.017 |
| UPDRS-III | 2.0 ± 2.6 (1) | 1.5 ± 2.1 (1) | 0.46 |
| II. Blood Metals | |||
| Mn (ng/ml) | 10.64 ± 3.0 (10) | 8.53 ± 2.11 (8.55) | 0.006 |
| Fe (μg/ml) | 558 ± 59 (559) | 496 ± 76 (510) | <10−4 |
| Cu (ng/ml) | 880 ± 127 (897) | 755 ± 137 (768) | 0.0006 |
| Pb (ng/ml) | 22.0 ± 19.6 (16.5) | 9.74 ± 8.64 (8.95) | <10−4 |
| K (μg/ml) | 2101 ± 399 (2136) | 1784 ± 350 (1675) | 0.003 |
| Cr (ng/ml) | 6.66 ± 5.26 (8.9) | 8.43 ± 20.8 (1.06) | 0.06 |
| III. MRI Measures | |||
| GP R1 | 0.88 ± 0.06 (0.87) | 0.87 ± 0.06 (0.87) | 0.86 |
| PUT R1 | 0.71 ± 0.05 (0.70) | 0.70 ± 0.05 (0.69) | 0.37 |
| CN R1 | 0.67 ± 0.08 (0.66) | 0.66 ± 0.05 (0.66) | 0.46 |
| AMY R1 | 0.55 ± 0.06 (0.53) | 0.54 ± 0.04 (0.54) | 0.74 |
| HIP R1 | 0.52 ± 0.05 (0.51) | 0.51 ± 0.04 (0.51) | 0.62 |
| OFGM R1 | 0.64 ± 0.04 (0.64) | 0.65 ± 0.04 (0.64) | 0.62 |
| OFWM R1 | 0.92 ± 0.07 (0.91) | 0.93 ± 0.08 (0.93) | 0.66 |
| PI | 109 ± 2 (109) | 109 ± 2 (109) | 0.46 |
*P-values indicate significant results at P < 0.05 using nonparametric Kruskal-Wallis tests
As part of the screening visit, detailed demographic information was taken from all subjects that included age, education, history of smoking, and history of current and/or past major medical/neurological disorders. All subjects were examined and ascertained to be free of any obvious neurological and movement deficits using the Unified PD Rating Scale-motor scores (UPDRS-III) with threshold score of <15 (Racette et al., 2012). All subjects had normal liver function, normal blood calcium, and magnesium levels, and no Fe deficiency. All welders underwent an orbital radiograph to rule out any metal fragments around the orbit. Written informed consent was obtained from all subjects in accordance with guidelines approved by the Internal Review Board/Human Subjects Protection Office of the Penn State Milton S. Hershey Medical Center.
Exposure assessment
Exposures were assessed by 2 questionnaires and by analysis of blood samples. The work history (WH, available upon request) questionnaire was designed to collect job information for the individual’s working lifetime, with an emphasis on characterizing welding and other jobs that would be associated with Mn exposure. An additional supplementary exposure questionnaire (SEQ, available upon request) focused on the 3-month period prior to the MRI and blood draw, and determined the time spent welding, the type of metal welded, and the various types of welding performed. Information on respirator use, confined space work, and use of ventilation also was collected. The primary exposure metrics derived from the SEQ were: hours welding, brazing, or soldering (HrsW, see details in Supplement 1) in the 90-day period preceding MRI and blood draw; and an estimate of the cumulative 90-day exposure to Mn (E90, see details in Supplement 1). Responses to the WH enabled an estimate of the cumulative lifetime years welding (YrsW) and also an estimate of cumulative exposure to inhaled Mn over the individual’s life (ELT, see details in Supplement 2).
Blood analysis
Whole blood was analyzed for a variety of metals by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) including Mn, Fe, K, Cu, Cr, and Pb. Digestion was performed by microwave methods using the Discovery SPD digestion unit (CEM, Matthews, North Carolina). After digestion, the samples were analyzed for trace minerals using the Thermo (Bremen, Germany) Element 2 SF-ICP-MS equipped with a concentric glass nebulizer and Peltier-cooled glass cyclonic spray chamber. Bulk mineral concentrations were determined by ICP-OES (Optical Emission Spectrometry) analysis on the Thermo iCAP equipped with a polypropylene cyclonic spray chamber.
MRI image acquisition and analysis
All images were acquired using Siemens 3 T scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) with an 8-channel head coil. First, high-resolution T1-weighted and T2-weighted images were acquired for anatomical segmentation and PI estimation. T1W images were collected using an MPRAGE sequence with Repetition Time (TR) = 1540 ms, Echo Time (TE) = 2.3 ms, FoV =256 × 256 mm, matrix = 256 × 256 mm, slice thickness = 1 mm, slice number = 176 (with no gap), and voxel spacing 1 × 1 × 1 mm. T2-weighted images were collected using a fast-spin-echo sequence with TR/TE = 2500/316, and the same spatial resolution as the T1W images. For whole brain fast T1 mapping, images were acquired using a spoiled gradient echo with 2 flip angles and transmit field (B1) correction. Image acquisition parameters for the T1 mapping were as follows: TR = 15 ms, TE = 1.45 ms, flip angles = 4/25, FoV = 250 × 250 mm, matrix = 160 × 160, slice thickness = 1 mm, slice number = 192 50% overlap, and voxel spacing = 1.56 × 1.56 × 1 mm; and for the B1 field mapping: TR = 1000 ms, TE = 14 ms, flip angles =45/60/90/120/135, FoV = 250 × 250 mm, matrix = 32 × 32, slice thickness = 5 mm, and slice number = 22.
Defining brain ROI
Bilateral basal ganglia structures [GP, putamen (PUT), caudate nucleus (CN)], amygdala (AMY), hippocampus (HIP), OFWM, and orbitofrontal gray matter (OFGM) were selected as ROIs based on literature that reported the effects of Mn exposure on movement, mood, and cognitive function mediated by these ROIs (Chang et al., 2009; Criswell et al., 2012; Dorman et al., 2006). The target ROIs (GP, PUT, CN, AMY, and HIP) were defined for each subject using automatic segmentation software (AutoSeg) (Gouttard et al., 2007; Joshi et al., 2004). The ROIs then were eroded by 1 voxel using a morphological operation in order to make sure that the segmented ROIs were within the anatomical ROIs. Unlike subcortical ROIs, frontal ROIs contain significant cortical folding, thus automatic segmentation was less useful in delineating OFGM and OFWM ROIs. For this reason, OFWM and OFGM were defined in a semiautomated fashion: these 2 ROIs were manually corrected by a skilled radiologist after the automatic segmentation using ITK-SNAP (Yushkevich et al., 2006) on the T1W images.
Estimation of pallidal index
First, T1W images were skull stripped and then a bias field correction was used to correct within-subject intensity variations caused by imperfect magnetic fields. Next, histogram-based intensity standardization was used to reduce magnetic field inhomogeneity between subjects (Ge et al., 2000; Nyúl et al., 2000; Sen et al., 2011). Subsequently, the GP and OFWM ROIs were mapped onto the intensity-corrected T1W images (co-registration). The mean intensities of the individual ROIs for each subject were calculated using a trimmed mean (5–95% percentile) to reduce possible segmentation error and imaging noise. The PI was derived from the ratio of GP T1W intensity to OFWM intensity [PI = (GP/OFWM) × 100] (Krieger et al., 1995).
Estimation of R1 values
First, whole brain T1 images were generated by the scanner using a published method (Venkatesan et al., 1998). ROIs were coregistered onto the T1 maps using an affine registration implemented in 3D Slicer (www.slicer.org; Rueckert et al., 1999). The R1 values of each ROI were calculated as 1/T1 in each voxel and averaged over the entire ROI using a trimmed mean (5–95% percentile), the same method as used for the T1W intensities.
Statistical Analysis
Matlab (Mathworks Inc., Natick Massachusetts) and SAS (Cary, North Carolina) were used to perform all statistical analyses. Right and left hemisphere MRI data were averaged. Kruskal-Wallis nonparametric analysis of variance was used for between-group comparisons of all demographic variables and blood metal levels. Rank analysis of covariance was used for between-group comparisons of MRI R1s and PI with adjustments of age, blood metal levels (Cr, Cu, Fe, K, and Pb), body mass index (BMI), hemoglobin (HGB), and respirator use (Quade, 1967). All post hoc tests involving multiple group comparisons were corrected using the stepdown Bonferroni method (Ludbrook, 1998). We report raw P-values but indicate whether the test result was significant after the correction for multiple comparisons. The comparisons of R1 values in the selected ROIs were exploratory in nature, and thus were not corrected for multiple comparisons. The SAS mixed model procedure was used to evaluate the relationship between R1 and HrsW in the selected ROIs. To test the associations between exposure and blood metals with MRI markers, Spearman correlation analyses were conducted for welders with adjustment of age. The data from controls were excluded from the correlation analyses because they had no welding exposure history, thus the measured blood metal levels and MRI measures could not be related to welding exposure. Statistical significance was defined by α = 0.05.
RESULTS
Demographics and Exposure History
There was no group difference in age (P = 0.51) but controls had significantly more years of education than did welders (P < 0.0001). Welders had significantly greater exposure metrics of HrsW, E90, YrsW, and ELT than did controls (Ps < 0.0001; Table 1-I). The a priori hypothesis that blood levels of Mn, Fe, Pb, Cu, and K blood levels would be greater in welders relative to controls was confirmed (Ps < 0.006; Table 1-II). Welders showed higher BMI and HGB values than controls (Ps < 0.031). In general, each welder performed multiple types of welding, but overall shield metal arc welding (SMAW), gas metal arc welding, and gas tungsten arc welding accounted for most of the welding done by subjects in this study. The most frequent base metal reported was mild steel, followed by stainless steel. When asked to identify the most common SMAW electrode used, the E6010 (rutile) and E7018 (basic) electrodes were noted.
MRI Measures and Their Association With Exposure Measurements
Overall, there were no statistically significant differences between welders and controls for PI or R1 in any ROI (Ps > 0.49, Table 1-III). In welders, R1 showed significant correlations with HrsW in all brain ROIs (Ps < 0.006). E90 was significantly correlated with R1 AMYG (R = 0.35, P = 0.045). R1 CN and PUT values showed significant correlations with YrsW, a long-term exposure metric (Ps < 0.042; Table 2).
TABLE 2.
Spearman Correlation Coefficients of Exposure Metrics With MRI Measures in Welders With Adjustment of Age
| ROIs | HrsW | E90 | YrsW | ELT |
|---|---|---|---|---|
| GP R1 | 0.57* | 0.16 | 0.24 | 0.07 |
| PUT R1 | 0.57* | 0.30 | 0.36* | 0.32 |
| CN R1 | 0.48* | 0.32 | 0.35* | 0.17 |
| AMY R1 | 0.48* | 0.35* | 0.30 | 0.11 |
| HIP R1 | 0.46* | 0.27 | 0.16 | 0.11 |
| OFWM R1 | 0.55* | 0.22 | 0.17 | 0.04 |
| OFGM R1 | 0.39* | 0.43* | 0.12 | 0.08 |
| PI | 0.18 | 0.32 | 0.14 | 0.07 |
*Significant results at P < 0.05
Post Hoc Analysis of R1 Values With Different Levels of Exposure History
Despite significant correlations between HrsW and R1 in basal ganglia (and other ROI structures), the relationship was nonlinear. Figure 1 shows a scatterplot of R1 in both the GP and PUT in relation to HrsW indicating significant second-order polynomial fits (Ps < 0.01). This suggests that R1 does not start to increase in the GP until HrsW exceeds ∼300 h. Similar curves were observed in the CN (data not shown).
FIG. 1.
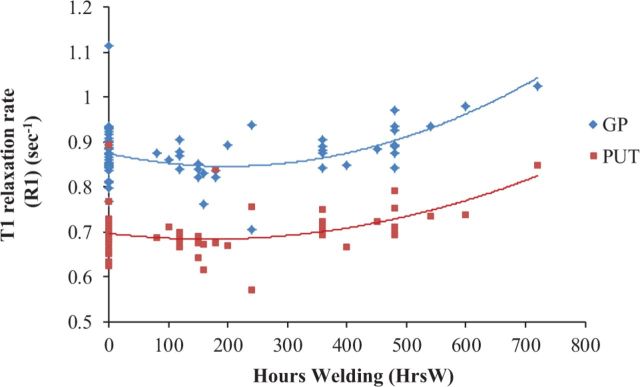
R1s in the GP (diamond-shaped dots) and PUT (square-shaped dots) as a function of welding hours (HrsW) [collapsed across welders (N = 35) and controls (N = 30)].
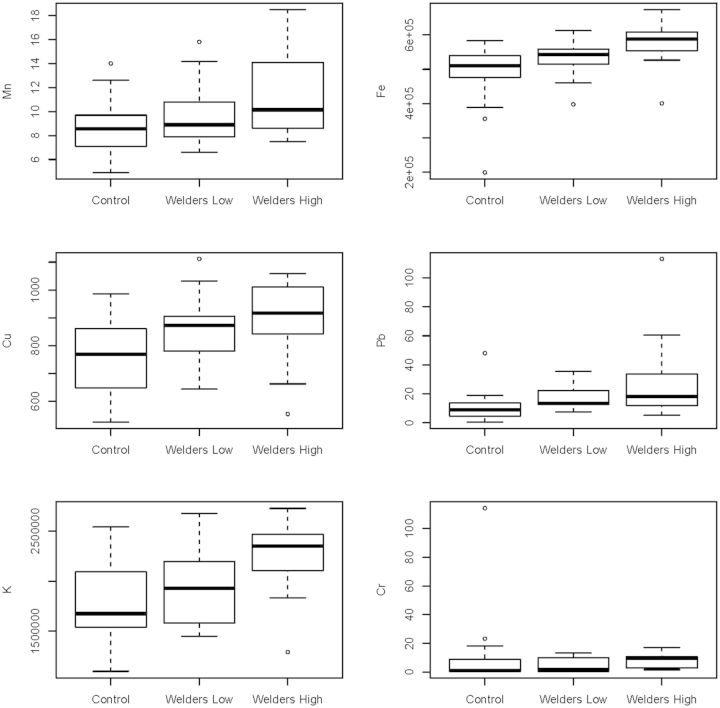
This nonlinearity suggested the creation of 3 post hoc exposure groups by dividing the welders based on HrsW. The resulting subgroups were: (1) Group A: controls (N = 30), (2) Group B: welders with <300 HrsW (N = 17), and (3) Group C: welders with more than 300 HrsW (N = 18). Table 3-I presents the exposure and demographic summaries and multiple comparisons among these post hoc groups. There was no significant age difference among the 3 groups (P = 0.77), but for all exposure measures the groups were consistently different with C > A, C > B (Ps < 0.0001) and for HrsW C > B > A (Ps < 0.0001), consistent with expectations. The comparisons of the blood measures showed that welders with a greater exposure had significantly higher concentrations than controls; this remained significant after correction for multiple comparisons (C > A, Ps < 0.0054; Table 3-II and Fig. 2).
TABLE 3.
Summary Statistics for: Demographics and Exposure Metrics (I); Blood Metals (II); and MRI Measures—R1 Regional Values and PI (III) in the 3 Post Hoc Subgroups
| A: Controls (N = 30) | B: Welders-Low (N = 17) | C: Welders-High (N = 18) | Significant Comparisons | |
|---|---|---|---|---|
| Mean ± SD (Median) | Mean ± SD (Median) | Mean ± SD (Median) | ||
| I. Demographics and exposure metrics | ||||
| Age (years) | 43.6 ± 11.5 (42.5) | 46.4 ± 11.5 (51) | 45.6 ± 11.2 (51) | |
| Education (years) | 16.2 ± 2.4 (16) | 12.9 ± 1.96 (13) | 12.7 ± 1.3 (12) | A > B*; A > C* |
| HrsW (h) | 0 ± 0 (0) | 152 ± 45 (150) | 457 ± 97 (480) | A < B < C* |
| YrsW (years) | 0 ± 0 (0) | 25.2 ± 12.1 (29) | 25.4 ± 10.7 (29.3) | A < B; A < C* |
| E90 (mg/m3—days) | 0.0027 ± 0 (0.0027) | 2.21 ± 1.17 (2.14) | 3.77 ± 1.86 (3.2) | A < B*; A < C* |
| ELT (mg/m3—years) | 0.0013 ± 0.0003 (0.0013) | 1.18 ± 0.77 (1.01) | 1.29 ± 0.78 (1.19) | A < B*; A < C* |
| AST (IU/l) | 35.7 ± 9.4 (35) | 33.5 ± 4.5 (36) | 39.4 ± 10.6 (38) | |
| ALT (IU/l) | 38.1 ± 16.9 (31) | 39.2 ± 10.2 (40) | 40.2 ± 23.7 (34.5) | |
| BMI | 25.3 ± 5.3 (26.5) | 30.2 ± 7.1 (27.8) | 27.2 ± 2.6 (27.2) | |
| Hemoglobin | 14.8 ± 0.8 (14.9) | 15.4 ± 0.8 (15.6) | 15.4 ± 1.1 (15.5) | |
| UPDRS-III | 1.5 ± 2.1 (1) | 2.1 ± 3.1 (1) | 1.9 ± 2.1 (1) | |
| II. Blood metals | ||||
| Mn (ng/ml) | 8.53 ± 2.11 (8.55) | 9.78 ± 2.5 (8.87) | 11.46 ± 3.3 (10.1) | A < C* |
| Fe (μg/ml) | 496 ± 76 (510) | 536 ± 53 (543) | 578 ± 573 (588) | A < C* |
| Cu (ng/ml) | 755 ± 137 (768) | 859 ± 116 (872) | 901 ± 136 (917) | A < C* |
| Pb (ng/ml) | 9.74 ± 8.64 (8.95) | 17.39 ± 7.59 (13.3) | 26.27 ± 25.96 (18) | A < B*; A < C* |
| K (μg/ml) | 1784 ± 351 (1675) | 1929 ± 397 (1930) | 2263 ± 336 (2352) | A < C*; B < C* |
| Cr (ng/ml) | 8.43 ± 20.8 (1.06) | 4.82 ± 5.08 (1.62) | 8.39 ± 4.94 (9.67) | A < C* |
| III. MRI measures | ||||
| GP R1 | 0.87 ± 0.06 (0.87) | 0.85 ± 0.05 (0.84) | 0.90 ± 0.05 (0.89) | B < C* |
| PUT R1 | 0.70 ± 0.05 (0.69) | 0.68 ± 0.06 (0.68) | 0.73 ± 0.04 (0.72) | A < C; B < C* |
| CN R1 | 0.66 ± 0.05 (0.66) | 0.66 ± 0.11 (0.64) | 0.68 ± 0.03 (0.67) | B < C |
| AMY R1 | 0.54 ± 0.04 (0.54) | 0.54 ± 0.09 (0.53) | 0.55 ± 0.02 (0.54) | |
| HIP R1 | 0.51 ± 0.04 (0.51) | 0.51 ± 0.06 (0.51) | 0.52 ± 0.02 (0.52) | |
| OFGM R1 | 0.65 ± 0.04 (0.64) | 0.63 ± 0.04 (0.62) | 0.65 ± 0.03 (0.65) | |
| OFWM R1 | 0.93 ± 0.08 (0.93) | 0.89 ± 0.08 (0.88) | 0.94 ± 0.04 (0.95) | B < C* |
| PI | 109 ± 2 (109) | 109 ± 2 (109) | 110 ± 3 (110) | |
Significant comparisons indicate pairwise comparisons at P < 0.05 and *comparisons that remain significant at P < 0.05 after correction for multiple group comparisons using the stepdown Bonferroni method.
FIG. 2.
Box plots for blood measures for the 3 post hoc subgroups.
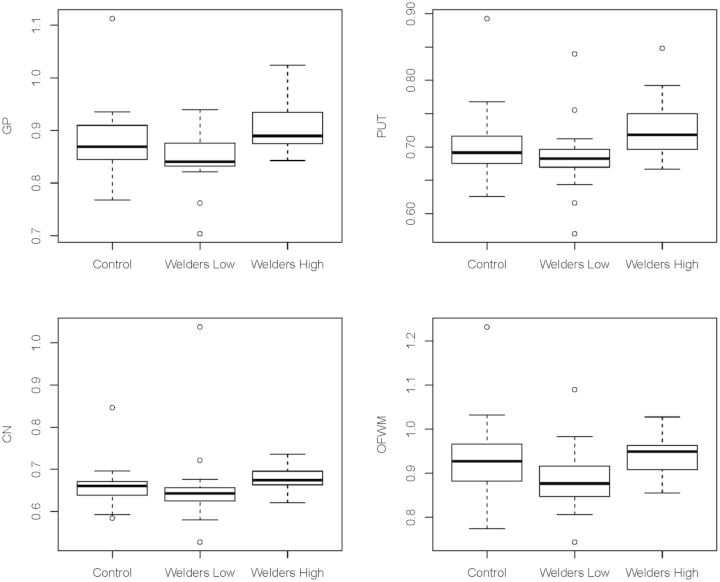
Table 3-III presents the MRI metrics for the 3 post hoc subgroups. Higher R1 values for the highly exposed welders (Group C) relative to the controls (Group A) were found in the PUT (P = 0.027). The highly exposed welders (Group C) had greater R1 measures than welders with lower exposure (Group B) in CN, PUT, GP, and OFWM (Ps < 0.027; Fig. 3). These results in R1 PUT, GP, and OFWM remained significant after correction for multiple comparisons. Notably, the PI was not significantly different for any of the comparisons (Ps > 0.26). Figure 4 is a plot of the R1 values versus HrsW in the 5 ROIs for Group C (welders with >300 HrsW). Above this exposure level, the slope between R1 and HrsW is statistically significant in all 5 regions with the GP having the highest sensitivity (i.e., greater slope; P = 0.0005). The slope of the relationship in the GP was significantly greater than in all other regions (Ps < 0.006), with a trend for significance compared with the PUT (P = 0.06).
FIG. 3.
Box plots for significantly different pairwise R1 comparisons among the 3 post hoc subgroups.
FIG. 4.
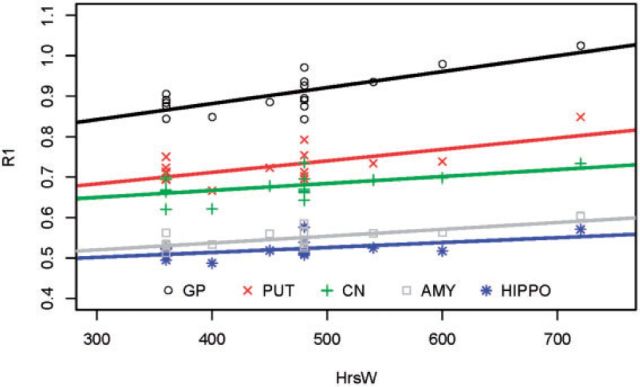
Linear relationships between R1 and HrsW for welders with >300 HrsW.
DISCUSSION
This study sought to examine whether Mn exposure through welding was associated with the T1 relaxation rates in a PA-based cohort. The results demonstrated that (1) there were significant nonlinear correlations (especially in the GP and PUT) between R1 values and HrsW in the 90-day period preceding MRI; (2) welders with higher exposure had elevated R1 values in the PUT when compared with controls, and in the GP, PUT, CN, and OFWM when compared with welders with lower exposure; and (3) at low exposure levels the PI lost sensitivity as a marker probably due to Mn accumulating in the OFWM.
All of the exposure metrics derived from the questionnaires and blood levels were greater for welders than controls. It is known that the primary exposures in many types of welding are to Fe, K, and Mn (Burgess, 1995). The mean blood Mn levels for both welders (10.6 ± 3.0 ng/ml) and controls (8.5 ± 2.1) were in reasonable agreement with general population values (9.0 ± 2.3 ng/ml) (Pleban and Pearson, 1979). Our average blood Mn level in welders is in good agreement with the mean level reported from a large European study of welders (i.e., 10.3 ng/ml) (Pesch et al., 2012).
The average blood Mn levels and PI values in our welders, however, were considerably lower compared with several other studies that reported both blood Mn levels and MRI data (e.g., blood Mn > 14.2 ng/ml and PI > 112) (Chang et al., 2009; Choi et al., 2007), suggesting that our welders probably had overall lower Mn exposure. Many welders in our study worked only intermittently during the 90-day exposure period (Table 3), and this was likely a factor in keeping blood Mn levels and MRI indices (PI & R1 values) low relative to other studies (Chang et al., 2009; Dorman et al., 2006). Note that a welder with 300 HrsW is approximately equivalent to what a half-time welder completely engaged in welding during the 90-day period would report. Some (protective) respirator usage also was reported by several of our welders, especially those with HrsW >300, and this also may have attenuated blood and MRI levels. Therefore, the wide range of exposure in our welders and their relatively low Mn exposure levels probably contributed to the lack of a significant difference in R1 values for all brain ROIs between welders and controls.
The inclusion of low-level Mn exposure subjects, however, was very illustrative. Welders with lower exposures (HrsW <300) were indistinguishable from controls (blood metal and R1 values), whereas the R1 value in the PUT of welders with over 300 HrsW was significantly different. R1s in the GP, PUT, CN, and OFWM of the highly exposed welders were greater than those of the low-level exposed welders after controlling for confounders such as other blood metal levels, Fe deficiency, age, BMI, and respirator use. This is consistent with a physiologically based pharmacokinetic (PBPK) study of Mn that predicted that the accumulation of Mn in the GP is essentially unchanged after inhalation exposures below 0.1 mg/m3 for 8 h/day over 90 days (Schroeter et al., 2011). This study is novel in that it provides evidence that PBPK stimulation may have a basis in reality with human subjects. Our study provides the first clinical evidence that is consistent with this PBPK stimulation. It is not straightforward, however, to estimate how 300 HrsW translates to air concentration. An estimate based on the average Mn time weighted average for welders of 0.27 mg/m3 for a fully engaged welder (HrsW ∼520), however, would lead to a comparable inhalation concentration of 0.16 mg/m3, in approximate agreement with the PBPK results. Thus, one would not expect to see much correlation between R1 and exposure until inhalation concentrations exceeded this level.
Interestingly, the R1s were better associated with HrsW (a simple welding-related time-weighted exposure metric) than with E90 probably because the potential for misclassification of the latter becomes greater as more information is incorporated. In addition, it was not feasible to measure the airborne Mn level in the work place, an important factor for estimating E90. Together, these factors could result in attenuation of any potential relationship between MRI and E90. Also, lower exposure welders showed somewhat lower R1s than the controls (although not significant). One might speculate that at the lower level exposure other metals (such as Fe) that are present in welding fumes may compete with Mn for metal transport systems.
The data in Figure 4 suggest that as Mn accumulates, it does so in all 5 ROIs interrogated but at a greater rate (per unit exposure) in the GP, consistent with the identification of the GP as the region with highest Mn accumulation in exposed populations (Spahr et al., 1996). This is consistent with the increased vulnerability of the GP to Mn-related toxicity and subsequent basal ganglia dysfunction and presentation of parkinsonism. It also provides additional data confirming that the PI is not sensitive to low levels of exposure. In our study, the PI values in welders were not correlated with HrsW or any exposure or blood metric, even for welders with higher exposures. This is consistent with the conjecture (Dorman et al., 2006) that the PI is confounded by Mn entering not only the GP, but also other brain areas including OFWM. Finally, our findings confirm that R1 estimates brain Mn tissue concentration more sensitively than PI. Specifically, the R1values in our study were consistently and positively correlated with HrsW in all ROIs, and appear to provide a more sensitive indicator for brain Mn accumulation than the PI. This is consistent with a report by Choi et al. (2007) that PI did not linearly increase with R1 when the exposure level was low. Unlike our study, Choi et al. (2007) reported that PI was correlated with both short-term and long-term exposure measures, possibly reflecting a higher Mn exposure level in their study. This suggests an important advantage of R1 over PI as a marker of brain Mn burden that may be particularly relevant to low-level environmental exposure scenarios in the public health domain.
The short-term (90-day) exposure metric of HrsW was correlated more strongly with R1s in welders than with longer-term exposure measures. This is consistent with an earlier finding reporting that R1 in GP was better correlated with the short-term than long-term cumulative exposure (Choi et al., 2007). This study, however, also found that R1s in CN and PUT were associated with the longer-term measure (YrsW). This result is consistent with the finding of Criswell et al. (2012) reporting that TIWI indices in CN and PUT were correlated with the cumulative long-term exposure hours. The exact reason why R1 in CN and PUT, but not other regions (Table 2) correlated with long-term Mn exposure is unclear. Further studies are needed to understand the Mn accumulation in brain in relation to short- and long-term exposures.
We found increased R1 in higher exposed welders compared with either controls (R1 PUT), or compared with lower-exposed welders (R1s in GP, PUT, CN, and OFWM) even after controlling for a number of potential confounders. This suggests that the group differences in R1s may be mainly due to the differences in Mn exposure level. Although R1 values were associated with Mn exposure, as noted earlier other metals such as Fe may also have affected this measure (Fitsanakis et al., 2010). Fe and Mn welding exposure is often correlated, and Fe levels are about 10-fold that of Mn (Flynn and Susi, 2009). Mn and Fe compete for common transporters (i.e., transferrin) to cross the BBB (Fitsanakis et al., 2010). In this regard, the lack of group difference between higher-exposed welders and controls in R1s, particularly in R1 GP, may be due, in part, to increased Fe levels by decreasing the transport of Mn into the brain despite the increased Mn concentration in blood. This is consistent with the fact that the GP is particularly enriched in Fe (Zhang et al., 2009).
It is worthwhile to note that the major limitation of our study (and prior ones) with active welders is the inability to measure actual brain content of these metals. As a result, it is not possible to make the direct link between Mn and other metal exposures to the elevated R1 in welders. Nevertheless, the current findings of the higher R1s in the higher-exposed welders compared with controls or lower-exposed welders, after controlling for a number of confounders (including blood metal levels other than Mn), support the Mn exposure-R1 association. This study of asymptomatic welders with relatively lower level Mn exposure is consistent with the notion that supports that R1 appears to be a more sensitive indicator of Mn accumulation in brain than PI. The finding of a nonlinear relationship between Mn exposure and brain accumulation is novel and needs to be further explored. These results may guide future studies and the development of occupation- and public health-related polices involving Mn exposure.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
We thank all the volunteers who participated in this study. In addition, we are indebted to many individuals who helped make this study possible including: Melissa Santos and Susan Kocher for subject coordination, recruitment, blood sample handling, and data entry; Pam Susi and Pete Stafford of CPWR; Mark Garrett, John Clark, and Joe Jacoby of the international Brotherhood of Boilermakers; Fred Cosenza and all members of the Safety Committee for the Philadelphia Building and Construction Trades Council; Ed McGehean of the Steamfitters Local Union 420; Jim Stewart of the Operating Engineers; Sean Gerie of the Brotherhood of Maintenance of Way Employees Division Teamsters Rail Conference; and Terry Peck of Local 520 Plumbers, Pipefitters and HVAC. The authors do not have any financial conflicts of interest to disclose.
Glossary
Abbreviations
- AMY
amygdala
- ANOVA
analysis of variance
- BBB
blood–brain–barrier
- CN
Caudate nucleus
- E90
cumulative 90-day exposure to Mn
- ELT
cumulative exposure to inhaled Mn over the individual’s life
- GP
Globus pallidus
- HIP
hippocampus
- HrsW
hours welding, brazing, or soldering in the 90-day period preceding MRI
- MRI
magnetic resonance imaging
- OFGM
orbitofrontal gray matter
- OFWM
orbitofrontal white matter
- PD
Parkinson’s disease
- PI
pallidal index
- PUT
Putamen
- R1
T1 relaxation rate
- ROIs
regions-of-interest
- T1
MRI longitudinal relaxation time
- T1WI
T1-weighted intensity
- TE
echo time
- TR
repetition time
- UPDRS
unified PD rating scale
- YrsW
cumulative lifetime years welding
FUNDING
R01 ES019672 from the National Institute of Environmental Health Sciences.
REFERENCES
- Baker M. G., Criswell S. R., Racette B. A., Simpson C. D., Sheppard L., Checkoway H., Seixas N. S. (2014a). Neurological outcomes associated with low-level manganese exposure in an inception cohort of asymptomatic welding trainees. Scand. J. Work Environ. Health 41, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. G., Simpson C. D., Stover B., Sheppard L., Checkoway H., Racette B. A., Seixas N. S. (2014b). Blood manganese as an exposure biomarker: state of the evidence. J. Occup. Environ. Hyg. 11, 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler R. M., Gysens S., Diamond E., Nakagawa S., Drezgic M., Roels H. A. (2006). Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 27, 315–326. [DOI] [PubMed] [Google Scholar]
- Burgess W. A. (1995) Recognition of health hazards in industry: a review of materials and processes. 2nd ed., Wiley, New York. [Google Scholar]
- Cersosimo M. G., Koller W. C. (2006). The diagnosis of manganese-induced parkinsonism. Neurotoxicology 27, 340–346. [DOI] [PubMed] [Google Scholar]
- Chang Y., Kim Y., Woo S.-T., Song H.-J., Kim S. H., Lee H., Kwon Y. J., Ahn J.-H., Park S.-J., Chung I.-S. (2009). High signal intensity on magnetic resonance imaging is a better predictor of neurobehavioral performances than blood manganese in asymptomatic welders. Neurotoxicology 30, 555–563. [DOI] [PubMed] [Google Scholar]
- Choi D. S., Kim E. A., Cheong H.-K., Khang H. S., Ryoo J. W., Cho J. M., Sakong J., Park I. (2007). Evaluation of MR signal index for the assessment of occupational manganese exposure of welders by measurement of local proton T1 relaxation time. Neurotoxicology 28, 284–289. [DOI] [PubMed] [Google Scholar]
- Criswell S. R., Perlmutter J. S., Huang J. L., Golchin N., Flores H. P., Hobson A., Aschner M., Erikson K. M., Checkoway H., Racette B. A. (2012). Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup. Environ. Med. 69, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A. W., Erikson K. M., Aschner M. (2004). Manganese neurotoxicity. Ann. N. Y. Acad. Sci. 1012, 115–128. [DOI] [PubMed] [Google Scholar]
- Dorman D. C., Struve M. F., Wong B. A., Dye J. A., Robertson I. D. (2006). Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol. Sci. 92, 219–227. [DOI] [PubMed] [Google Scholar]
- Fitsanakis V. A., Zhang N., Garcia S., Aschner M. (2010). Manganese (Mn) and iron (Fe): interdependency of transport and regulation. Neurotox. Res. 18, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M. R., Susi P. (2009). Manganese, iron, and total particulate exposures to welders. J. Occup. Environ. Hyg. 7, 115–126. [DOI] [PubMed] [Google Scholar]
- Gallez B., Demeure R., Baudelet C., Abdelouahab N., Beghein N., Jordan B., Geurts M., Roels H. A. (2001). Non-invasive quantification of manganese deposits in the rat brain by local measurement of NMR proton T1 relaxation times. Neurotoxicology 22, 387–392. [DOI] [PubMed] [Google Scholar]
- Ge Y., Udupa J. K., Nyul L. G., Wei L., Grossman R. I. (2000). Numerical tissue characterization in MS via standardization of the MR image intensity scale. J. Magn. Reson. Imag. 12, 715–721. [DOI] [PubMed] [Google Scholar]
- Gouttard S., Styner M., Joshi S., Smith R. G., Hazlett H. C., Gerig G. (2007). Subcortical structure segmentation using probabilistic atlas priors. Med. Imag. Int. Soc. Optics Photon. 65122J1–65122J11. [Google Scholar]
- Joshi S., Davis B., Jomier M., Gerig G. (2004). Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage 23, S151–S160. [DOI] [PubMed] [Google Scholar]
- Kesselring J., Miller D. H., Robb S. A., Kendall B. E., Moseley I. F., Kingsley D., du Boulay E. P., McDonald W. I. (1990). Acute disseminated encephalomyelitis. MRI findings and the distinction from multiple sclerosis. Brain 113(Pt 2), 291–302. [DOI] [PubMed] [Google Scholar]
- Krieger D., Krieger S., Theilmann L., Jansen O., Gass P., Lichtnecker H. (1995). Manganese and chronic hepatic encephalopathy. Lancet 346, 270–274. [DOI] [PubMed] [Google Scholar]
- Ludbrook J. (1998). Multiple comparison procedures updated. Clin. Exp. Pharmacol. Physiol. 25, 1032–1037. [DOI] [PubMed] [Google Scholar]
- Newland M. C., Cox C., Hamada R., Oberdörster G., Weiss B. (1987). The clearance of manganese chloride in the primate. Fund. Appl. Toxicol. 9, 314–328. [DOI] [PubMed] [Google Scholar]
- Nyúl L. G., Udupa J. K., Zhang X. (2000). New variants of a method of MRI scale standardization. IEEE Trans. Med. Imag. 19, 143–150. [DOI] [PubMed] [Google Scholar]
- Pal P. K., Samii A., Calne D. (1998). Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20, 227–238. [PubMed] [Google Scholar]
- Pesch B., Weiss T., Kendzia B., Henry J., Lehnert M., Lotz A., Heinze E., Käfferlein H. U., Van Gelder R., Berges M. (2012). Levels and predictors of airborne and internal exposure to manganese and iron among welders. J. Expo. Sci. Environ. Epidemiol. 22, 291–298. [DOI] [PubMed] [Google Scholar]
- Pleban P. A., Pearson K. H. (1979). Determination of lead in whole blood and urine using Zeeman effect flameless atomic absorption spectroscopy. Anal. Lett. 12, 935–950. [DOI] [PubMed] [Google Scholar]
- Quade D. (1967). Rank analysis of covariance. J. Am. Stat. Assoc. 62, 1187–1200. [Google Scholar]
- Racette B. A., Criswell S. R., Lundin J. I., Hobson A., Seixas N., Kotzbauer P. T., Evanoff B. A., Perlmutter J. S., Zhang J., Sheppard L. (2012). Increased risk of parkinsonism associated with welding exposure. Neurotoxicology 33, 1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette B., Tabbal S., Jennings D., Good L., Perlmutter J., Evanoff B. (2005). Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology 64, 230–235. [DOI] [PubMed] [Google Scholar]
- Rueckert D., Sonoda L. I., Hayes C., Hill D. L., Leach M. O., Hawkes D. J. (1999). Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imag. 18, 712–721. [DOI] [PubMed] [Google Scholar]
- Schroeter J. D., Nong A., Yoon M., Taylor M. D., Dorman D. C., Andersen M. E., Clewell H. J. (2011). Analysis of manganese tracer kinetics and target tissue dosimetry in monkeys and humans with multi-route physiologically-based pharmacokinetic models. Toxicol. Sci. 120, 481–498. [DOI] [PubMed] [Google Scholar]
- Sen S., Flynn M. R., Du G., Tröster A. I., An H., Huang X. (2011). Manganese accumulation in the olfactory bulbs and other brain regions of “asymptomatic” welders. Toxicol. Sci. 121, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr L., Butterworth R. F., Fontaine S., Bui L., Therrien G., Milette P. C., Lebrun L. H., Zayed J., Leblanc A., Pomier-Layrargues G. (1996). Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 24, 1116–1120. [DOI] [PubMed] [Google Scholar]
- Taube F. (2013). Manganese in occupational arc welding fumes—aspects on physicochemical properties, with focus on solubility. Ann. Occup. Hyg. 57, 6–25. [DOI] [PubMed] [Google Scholar]
- Venkatesan R., Lin W., Haacke E. M. (1998). Accurate determination of spin-density and T1 in the presence of RF-field inhomogeneities and flip-angle miscalibration. Magn. Reson. Med. 40, 592–602. [DOI] [PubMed] [Google Scholar]
- Williams M., Todd G. D., Roney N., Crawford J., Coles C., McClure P. R., Garey J. D., Zaccaria K., Citra M. (2012). Toxicological Profile for Manganese. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles, Atlanta, GA, USA. [PubMed] [Google Scholar]
- Yilmaz A., Yurdakoc M., Işik B. (1999). Influence of transition metal ions on NMR proton T1 relaxation times of serum, blood, and red cells. Biol. Trace Elem. Res. 67, 187–193. [DOI] [PubMed] [Google Scholar]
- Yokel R. A. (2009). Manganese flux across the blood–brain–barrier. NeuroMol. Med. 11, 297–310. [DOI] [PubMed] [Google Scholar]
- Yushkevich P. A., Piven J., Hazlett H. C., Smith R. G., Ho S., Gee J. C., Gerig G. (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128. [DOI] [PubMed] [Google Scholar]
- Zhang N., Fitsanakis V. A., Erikson K. M., Aschner M., Avison M. J., Gore J. C. (2009) A model for the analysis of competitive relaxation effects of manganese and iron in vivo. NMR Biomed. 22, 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.