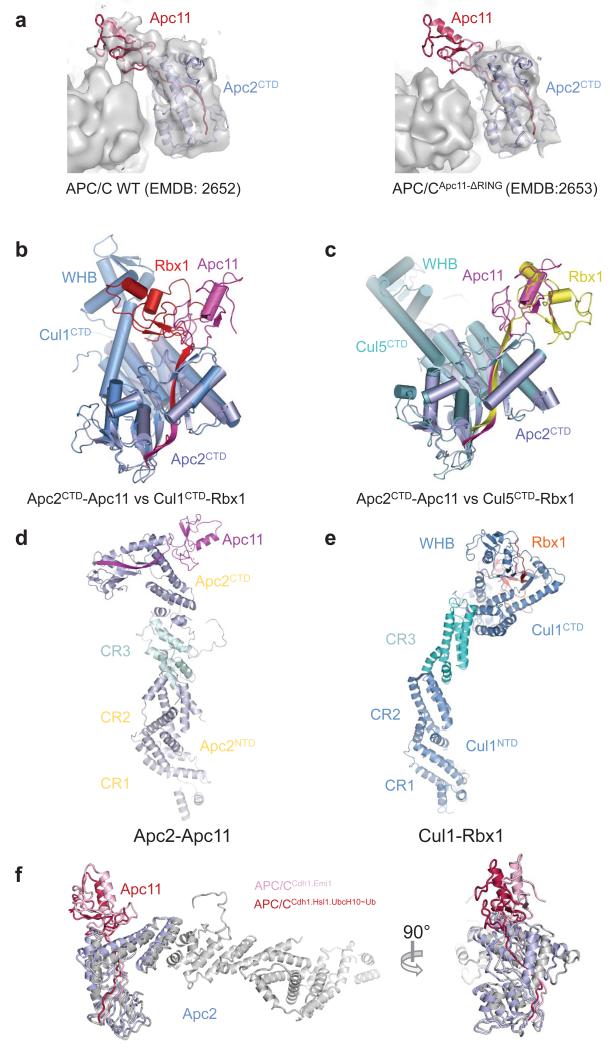
Extended Data Figure 5. The position of Apc11RING in the APC/C is more similar to Rbx1RING of activated cullin-Rbx1 structures.
(a) Identification of Apc11 in apo APC/C. Left panel: EM density map for apo APC/C with the coordinates of Apc2CTD-Apc11 fitted (from APC/CCdh1.Emi1 structure). Right panel. EM density for APC/CApc11-ΔRING. The difference density corresponds to Apc11RING. EM density maps from 4. (b) Superimposed Apc2CTD onto Cul1CTD (PDB: 1LDK) 61. (c) Superimposed Apc2CTD onto Cul5CTD (PDB: 3DQV) 27. In the inactive conformation of Cul1-Rbx1, Rbx1RING packs against WHB. In APC/CCdh1.Emi1 the location of Apc11RING remains in contact with Apc2CTD but has rotated ~180° relative to inactive CRL structures being similar to the swung out conformation of Rbx1RING of neddylated and activated Cul5-Rbx1 27. (d and e) The relative orientation of Apc2NTD and Apc2CTD is also dramatically different from Cul1 (Ref. 61). This is due to a 70° rotation within cullin repeat 3 (between helices A-B and C-D-E), and a ~20° rotation around the 4HB - cullin repeat 3 interface. Similar less pronounced structural variations are observed within the CRL family. (d) Apc2-Apc11 (this study). (e) Cul1-Rbx1 (PDB: 1LDK) 61. (f) The position of the Apc2CTD-Apc11 module differs slightly about the Apc2NTD-Apc2CTD interface between APC/CCdh1.Emi1 and APC/CCdh1.Hsl1.UbcH10-Ub.