Abstract
Background
Type 4 cardiorenal syndrome (CRS) refers to the cardiac injury induced by chronic kidney disease. We aimed to assess oxidative stress and cardiac injury in patients with type 4 CRS, determine whether the antioxidant apocynin attenuated cardiac injury in rats with type 4 CRS, and explore potential mechanisms.
Methods and Results
A cross-sectional study was conducted among patients with type 4 CRS (n=17) and controls (n=16). Compared with controls, patients with type 4 CRS showed elevated oxidative stress, which was significantly correlated with cardiac hypertrophy and decreased ejection fraction. In vivo study, male Sprague-Dawley rats underwent 5/6 subtotal nephrectomy and sham surgery, followed with apocynin or vehicle treatment for 8 weeks. Eight weeks after surgery, the 5/6 subtotal nephrectomy rats mimicked type 4 CRS, showing increased serum creatinine, cardiac hypertrophy and fibrosis, and decreased ejection fraction compared with sham-operated animals. Cardiac malondialdehyde, NADPH oxidase activity, fibroblast growth factor-2, and extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation increased significantly in the 5/6 subtotal nephrectomy rats. These changes were significantly attenuated by apocynin. In vitro study showed that apocynin reduced angiotensin II–induced NADPH oxidase–dependent oxidative stress, upregulation of fibroblast growth factor-2 and fibrosis biomarkers, and ERK1/2 phosphorylation in cardiac fibroblasts. Importantly, the ERK1/2 inhibitor U0126 reduced the upregulation of fibroblast growth factor-2 and fibrosis biomarkers in angiotensin II–treated fibroblasts.
Conclusions
Oxidative stress is a candidate mediator for type 4 CRS. Apocynin attenuated cardiac injury in type 4 CRS rats via inhibiting NADPH oxidase–dependent oxidative stress-activated ERK1/2 pathway and subsequent fibroblast growth factor-2 upregulation. Our study added evidence to the beneficial effect of apocynin in type 4 CRS.
Keywords: cardiac remodeling, cardiorenal syndrome, extracellular signal-regulated kinase 1/2, fibroblast growth factor, pharmacology
The heart and kidneys have a complicated and bidirectional interrelationship. The impaired function of 1 organ usually has a detrimental effect on the other, which in turn injures the function and structure of both organs. This interrelationship has been defined as cardiorenal syndrome (CRS).1 Type 4 CRS refers to the injury of cardiovascular structure and function in the setting of chronic kidney disease (CKD) and is also called chronic renocardiac syndrome.1 Previous studies showed that even mild impairment of renal function was associated with significantly elevated morbidity and mortality associated with cardiovascular diseases.2 Cardiovascular diseases, especially heart failure, are the major causes of death in CKD patients.3 The prevalence of CKD worldwide is estimated to be 8% to 16%,4 and the high incidence of type 4 CRS has a heavy burden on public health.3,5 Although there are new advances in clinical treatment for both cardiovascular and renal diseases, the treatment for type 4 CRS remains a challenge.6
The mechanism of type 4 CRS is complicated and involves many potential candidate mediators. Increasingly, findings suggest that oxidative stress may play an important role in cardiac and renal impairments in type 4 CRS.7 Oxidative stress is a prominent feature of CKD, which can be monitored with oxidative stress indicators or inducers, including malondialdehyde (MDA), superoxide dismutase (SOD), and asymmetric dimethylarginine and advanced oxidation protein products.8–10 In addition, both systemic and cardiac angiotensin II (Ang II) levels increase significantly in CKD.11 The renin-angiotensin-aldosterone system (RAAS) is activated in patients with CKD and involved in inducing oxidative stress and cardiac impairment.12 Oxidative stress is gradually recognized as a causal factor for cardiovascular diseases induced by CKD. Therefore, inhibition of oxidative stress with antioxidants may be a promising treatment strategy for type 4 CRS.
Apocynin is an assembly inhibitor of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase [NOX]) and widely used as an antioxidant in disease models in which oxidative stress is involved.13 Apocynin shows protective effects on kidneys and heart through the reduction of oxidative stress.14,15 However, whether inhibiting oxidative stress with apocynin can improve cardiac injury in type 4 CRS is still unclear.
In the present study, we sought to determine the relationship between oxidative stress level and cardiac injury in patients with type 4 CRS and to explore whether apocynin could reduce oxidative stress and attenuate cardiac injury in a rat model of type 4 CRS. The extracellular signal-regulated kinase 1/2 (ERK1/2) pathway can be activated by differential oxidative stress inducers, including advanced glycation end products and Ang II, and ERK1/2 is involved in the deleterious effects of these inducers on cardiac myocytes and fibroblasts.16,17 In addition, we18 and others’19 previously found that cardiac fibroblast growth factor (FGF)-2 is significantly upregulated in cardiac nonmyocytes by prohypertrophic factors, including Ang II, endothelin-1, and isoproterenol, and contributes to cardiac hypertrophy and fibrosis. However, it is still not clear whether FGF-2 is implicated in type 4 CRS. To explore potential mechanisms, we investigated the involvement of the ERK1/2 pathway and the role of FGF-2 in type 4 CRS.
Methods
Study Population and Data Collection
Patients admitted to Sun Yat-sen Memorial Hospital of Sun Yat-sen University for primary CKD during June to December in 2013 were enrolled in this cross-sectional study. Type 4 CRS is defined as cardiac abnormalities such as decreased cardiac function in the setting of primary CKD.1 In this study, patients with both moderate to severe CKD (stage 3 to 5) and heart failure were included in the type 4 CRS group. The patients had previously diagnosed primary CKD. Moderate to serious CKD was diagnosed when the estimated glomerular filtration rate was <60 mL/min as assessed using the Cockcroft–Gault formula. The diagnosis of heart failure was made according to the ESC Heart Failure Guidelines 2012.20 Patients with New York Heart Association class II through IV were classified as having heart failure based on medical history, symptoms, signs, and echocardiographic results. Exclusion criteria were acute renal failure, kidney transplantation, nephrotic syndrome, obvious chronic or acute cardiac abnormalities before CKD, neoplasm, severe hepatopathy, infectious diseases, acute or chronic inflammatory diseases. Patients who were taking immunosuppressive agents and classic antioxidants, such as carotenoid, vitamin C, or vitamin E, were also excluded. Among the patients enrolled, 17 patients were classified as having type 4 CRS. Patients without documented renal abnormalities and heart failure were classified as controls. A total of 16 controls were enrolled, but these controls had mild to moderate hypertension. The study conformed to the Helsinki Declaration. The Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University approved the protocol. Written informed consent was obtained from all patients.
On admission, medical history, demographic (age and sex), anthropometric (weight, height, and blood pressure) and biochemical parameters, and echocardiographic results were recorded. Previous studies found that RAAS inhibitors and β-blockers had inhibitory effects on oxidative stress.12,21 Therefore, the rate of use of RAAS inhibitors or β-blockers in both groups was recorded.
Venous blood samples for biochemical tests were drawn after overnight fasting. SOD, an important antioxidant enzyme, was tested to assess oxidative stress level. Plasma and erythrocyte SOD activity was measured with use of an assay kit (Cayman Chemical). The absorbance at 450 nm was recorded by using a Wallac Victor 2 multilabel counter (Perkin Elmer Life Sciences). Serum N-terminal pro-brain natriuretic peptide, commonly used in heart failure diagnosis, was detected with the use of an electrochemiluminescence immunoassay (Elecsys proBNP assay, Roche Diagnostics Corporation). For measurement of estimated glomerular filtration rate, serum creatinine was tested with the use of an automatic biochemical analyzer (7170A; HITACHI). Urea and high-sensitivity C-reactive protein were also measured with an automatic biochemical analyzer. Plasma Ang II level was tested with radioimmunoassay kits (Beijing North Institute of Biological Technology, Beijing, China). Echocardiography was performed to measure patients’ cardiac structural and functional changes with a 2.5-MHz transducer (Vivid 3; GE VingMed Ultrasound). Left ventricular posterior wall thickness at diastole (LVPWd), interventricular septum depth (IVSD), left ventricular end-diastolic diameter (LVEDD), and left ventricular ejection fraction (EF) were recorded.
Animal Model
Animal experiments were approved by the Animal Experimental Ethics Committee of Sun Yat-sen University and conducted in accordance with the “Guidelines for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
Male Sprague-Dawley rats obtained from Sun Yat-sen University weighing 160±20 g were housed in an environment-controlled room at 24±1°C with a 12-hour light/dark cycle and fed tap water and rodent chow. Animals were randomly divided into a sham-operated group (n=10), a 5/6 subtotal nephrectomy (STNx) group (n=10), and an STNx+apocynin group (n=10). A 2-step STNx was described previously as a model of CKD.22 Briefly, rats were anaesthetized with 40 mg/kg ketamine and 5 mg/kg xylazine (intraperitoneal injection), and the adequacy of anesthesia was determined by loss of response to the pinching of the skin of the abdomen, toes, or tails. The body temperature maintained at 37°C by using an electrical warming pad. The artery of left kidney was temporarily occluded, and then the upper and lower poles of this kidney were ligated and excised. In this way, one-third of the left kidney remained. Buprenorphine (0.03 mg/kg, subcutaneous injection twice daily for 3 days) was used for postoperative analgesia. After a 1-week recovery period, the right kidney was exposed and removed after ligation of the renal pedicle. Sham-operated rats underwent similar surgery but only the renal envelops were removed. Rats in the STNx+apocynin group were fed apocynin (Sigma-Aldrich) in drinking water (1.5 mmol/L) for 8 weeks.23 At baseline and weeks 4 and 8 after surgery, blood samples were collected through the tail vein for measurement of creatinine. Circulating Ang II at week 8 after surgery was detected with use of the radioimmunoassay kits (Beijing North Institute of Biological Technology).
Measurement of Blood Pressure and Heart Rate
At baseline and weeks 4 and 8 after surgery, systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were measured with a tail-cuff device (BP-98A; Softron). Conscious rats were placed into a restrainer with an electrical warming pad for 20 minutes, and all rats were trained to become accustomed to this process for 1 week before measurement. To avoid circadian variations of blood pressure and heart rate, all measurements were carried out between 8:00 and 11:00 am. At least 3 measurements of each rat were recorded at intervals of 1 to 2 minutes, and mean values of blood pressure and heart rate were calculated.
Echocardiography
Transthoracic echocardiography (IU22; Philips) was performed at week 8 after surgery. Pentobarbital (40 mg/kg intraperitoneally) was used for anesthesia. The following parameters were recorded: LVPWd, LVEDD, left ventricular end-systolic diameter (LVESD), left ventricular fraction shortening, and EF.
Histological Analysis
Rats were anesthetized with 1% pentobarbital (100 mg/kg intraperitoneally) and killed at the end of week 8. Body weight and left ventricular weight were measured to assess the ratio of left ventricular weight to body weight. Hearts were fixed with 4% paraformaldehyde and embedded in paraffin. Left ventricular sections were stained with Masson reagent for detecting fibrosis. To evaluate the degree of myocardial fibrosis, 10 fields of each section were randomly selected. The cardiac fibrosis volume fractions were calculated as the ratio of aniline blue–stained fibrosis areas to total myocardium areas with Image Pro-plus 5.0 software (Media Cybernetics).
Measurement of Cardiac MDA
Harvested hearts were stored at −80°C for Western blot analysis and the measurement of oxidative stress. Cardiac oxidative stress was determined by the measurement of cardiac MDA.9 Briefly, homogenates of left ventricular tissue were centrifuged at 1600 g for 10 minutes at 4°C. The levels of MDA in the supernatant were measured with thiobarbituric acid reaction by using a commercial kit (Beyotime Biological).24 The absorbency was detected with a multimode microplate reader (Spectra Max M5; Molecular Devices).
Cell Culture and Treatment
One- to 3-day-old neonatal Sprague-Dawley rats were killed by decapitation. The hearts were tore into small pieces and predigested by 0.125% trypsogen for 5 minutes and then digested with 0.06% collagenase-II for 2 hours in a shaker at 37°C. Collected cells were plated onto a culture dish for 45 minutes. Then, unattached cells were removed. The remained cardiac fibroblasts were cultured in high glucose (4500 mg/L) Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum in a humidified incubator with 5% CO2 at 37°C. The purity of cardiac fibroblasts was greater than 98% as determined by positive staining for vimentin and negative staining for von Willebrand factor. Cells were cultured in serum-free DMEM for 24 hours before treatment. Cells were treated with (1) dimethyl sulfoxide (DMSO) (1 μL, Sigma-Aldrich) alone, (2) Ang II (100 nmol/L, Sigma-Aldrich), (3) apocynin (100 μmol/L) alone, (4) Ang II (100 nmol/L)+apocynin (100 μmol/L), (5) ERK1/2 inhibitor U0126 (10 μmol/L, Cell Signaling Technology), or (6) Ang II (100 nmol/L)+U0126 (10 μmol/L). Apocynin and U0126 were dissolved in DMSO and added to cells for 1 hour before the stimulation of Ang II. Cells were treated with Ang II for 24 hours before detection of the expressions of procollagen I, procollagen III, transforming growth factor (TGF)-β and FGF-2.
Reactive Oxygen Species Assay
We determined intracellular reactive oxygen species (ROS) in cardiac fibroblasts by detecting superoxide anion with dihydroethidium (Molecular Probes, Invitrogen). Briefly, cells were treated with Ang II (100 nmol/L) with or without apocynin (100 μmol/L) for 2 hours and then incubated with dihydroethidium (10 μmol/L) for 30 minutes. Florescence was observed with a fluorescence microscope (DMI3000 B; Leica), and fluorescence intensities were detected with a multimode microplate reader.
Mitochondrial ROS production was measured by using MitoSox Red, a fluorescent probe specific for mitochondria ROS (Invitrogen). After treatment, cardiac fibroblasts were incubated with 3 μmol/L MitoSox Red for 30 minutes at 37°C. Florescence was observed with a fluorescence microscope, and fluorescence intensities were detected with a multimode microplate reader. Results were expressed as relative fluorescence intensity normalized to controls.
Measurement of NOX Activity
We used a lucigenin-enhanced chemiluminescence assay kit (Genmed Scientifics) to assess NOX activity. Briefly, the cardiac tissues and harvested cells were lysed and sonicated on ice. NOX activity was detected according to the manufacturer’s instructions. Chemiluminescence readings were normalized to total protein level. The final results were expressed as relative NOX activity normalized to controls.
Western Blot Analysis
The protein samples obtained from heart extracts and cell lysates were mixed with loading buffer and boiled at 95°C for 5 minutes. Boiled samples were separated on 10% to 12% SDS–polyacrylamide gels and proteins were transferred to PVDF membranes. The membranes were then incubated with primary antibodies: anti–TGF-β antibody, anti–total ERK1/2 antibody, anti–phospho-ERK1/2 antibody, anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (all from Cell Signaling Technology; dilution, 1:1000), anti–procollagen I antibody, anti–procollagen III antibody, or anti–FGF-2 antibody (all from Santa Cruz Biotechnology; dilution, 1:200) in Tris-buffered saline and Tween 20 containing 5% (w/v) bovine serum albumin (antibody buffer) overnight at 4°C. The membranes were then washed and incubated with horseradish peroxidase–linked secondary antibody (Cell Signaling Technology; dilution, 1:1000) and then visualized with enhanced chemiluminescence (Thermo Fisher Scientific). The densities of the bands were analyzed semiquantitatively and normalized with respect to GAPDH by image software (Thermo).
Statistical Analysis
Normal distribution data were expressed as mean±SD, and non-normal distribution data were expressed as median with interquartile range. Comparisons between 2 groups were performed with t test or Mann–Whitney U test. Repeated-measures analysis was used to examine overall differences in blood pressure, heartbeat, and serum creatinine in rats over time among groups. One-way ANOVA followed by a Bonferroni comparison test was used to compare data between multiple groups. Categorical data were compared with use of the χ2 test. Partial correlation analysis was used to assess the correlations between SOD level and echocardiographic data in patients with type 4 CRS and controls after controlling for age, sex, and weight. All the tests were performed by using SPSS version 13.0 (SPSS Inc). Statistical differences with a 2-tailed P value <0.05 were considered to be statistically significant.
Results
Oxidative Stress Was Significantly Associated With Cardiac Remodeling and Dysfunction in Patients With Type 4 CRS
A total 17 patients with type 4 CRS and 16 controls were included in the study. The characteristics of the 2 groups are shown in Table1. The 2 groups had no difference in sex, weight, DBP, and the rate of use of β-blockers or RAAS inhibitors. Patients with type 4 CRS were older and had higher SBP values than did the controls. Patients with type 4 CRS showed significantly elevated serum creatinine, urea, Ang II, and high-sensitivity C-reactive protein levels and lower estimated glomerular filtration rates compared with controls. As expected, patients with type 4 CRS displayed higher N-terminal pro-brain natriuretic peptide and lower EF than controls. Echocardiographic results showed elevated LVPWd and IVSD in patients with type 4 CRS compared with controls, indicating remarkably cardiac remodeling. Patients with type 4 CRS also showed increased LVEDD. In addition, increased oxidative stress level was detected in patients with type 4 CRS as suggested by decreased serum SOD level compared with controls (93±27 versus 131±20 U/mL, P<0.05). Partial correlation analysis (Table2) found that SOD level was inversely correlated with cardiac remodeling and positively correlated with EF, after controlling for age, sex, and weight. These findings indicated that increased oxidative stress may be an important factor related to the cardiac remodeling and dysfunction in patients with type 4 CRS.
Table 1.
Comparisons Between Patients With Type 4 CRS and Controls
| Variables | CRS (n=17) | Controls (n=16) |
|---|---|---|
| Age, y | 70±11 | 62±9* |
| Female/male | 8/9 | 7/9 |
| Weight, kg | 58+14 | 63+17 |
| SBP, mm Hg | 143±21 | 129±14* |
| DBP, mm Hg | 76±8 | 79±11 |
| β-Blockers or RAAS inhibitors | 17/17 | 14/16 |
| Biochemical data | ||
| Urea, mmol/L | 18.2±9.1 | 4.8±1.3* |
| Creatinine, mg/dL | 458±291 | 81±13* |
| eGFR, mL/min | 34.2±17.7 | 84.44±5.3* |
| hs-CRP, mg/L | 28.3 (3.8 to 98.2) | 2.7 (1.3 to 3.5)* |
| NT-proBNP, pg/mL | 25 944 (4407 to 34 787) | 101 (49 to 219)* |
| Ang II, ng/L | 59.4 (46.1 to 84.4) | 39.8 (32.8 to 57.2)* |
| SOD, U/mL | 93±27 | 131±20* |
| Echocardiographic data | ||
| LVPWd, mm | 11.1±2.3 | 8.9±1.1* |
| IVSD, mm | 11.5±2.4 | 9.1±1.1* |
| LVEDD, mm | 57.2±9.2 | 46.2±2.2* |
| EF, % | 36.2±9.4 | 67.3±7.1* |
Data were expressed as mean±SD or median (interquartile range). CRS indicates cardiorenal syndrome; Ang II indicates angiotensin II; SBP, systolic blood pressure; DBP, diastolic blood pressure; RAAS, renin-angiotensin-aldosterone system; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro-brain natriuretic peptide; SOD, superoxide dismutase; LVPWd, left ventricular posterior wall thickness at diastole; IVSD, interventricular septum depth; LVEDD, left ventricular end-diastolic diameter; EF, ejection fraction.
P<0.05 vs controls.
Table 2.
Partial Correlation Analysis Between SOD and Echocardiographic Parameters in Patients With Type 4 Cardiorenal Syndrome and Controls
| SOD | P Value | |
|---|---|---|
| LVPWd | −0.315 | 0.048 |
| IVSD | −0.378 | 0.036 |
| LVEDD | 0.102 | 0.532 |
| EF | 0.370 | 0.019 |
The partial correlation analysis was performed after adjustment for age, weight, and sex. SOD indicates superoxide dismutase; LVPWd, left ventricular posterior wall thickness at diastole; IVSD, interventricular septum depth; LVEDD, left ventricular end-diastolic diameter; EF, ejection fraction.
Apocynin Attenuated Cardiac Remodeling, Interstitial Fibrosis, and Cardiac Dysfunction in STNx Rats
Figure1 shows the results of blood pressure, heart rate, and serum creatinine in rats at baseline and weeks 4 and 8 after surgery. At 8 weeks after surgery, STNx rats showed significant increases in SBP and DBP but no significant change in heart rate (Figure1A through 1C). STNx also resulted in significantly higher levels of serum creatinine compared with sham surgery (Figure1D). Treatment with apocynin attenuated the increases in SBP and DBP but had no significant effect on the increased serum creatinine in STNx rats. Both STNx and apocynin treatment had no significant effect on heart rate. At week 8 after surgery, there was no significant difference in survival rate between STNx rats treated with and those not treated with apocynin (Table3).
Figure 1.
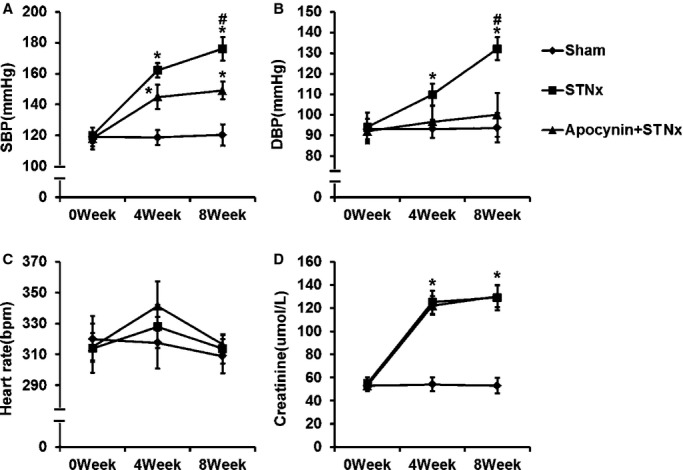
The effects of apocynin on blood pressure, heart rate and serum creatinine in rats at baseline, and the 4th and 8th week after subtotal nephrectomy. A through C, Apocynin reduced the elevated SBP and DBP in STNx rats. STNx and apocynin had no significant effect on heart rate. D, The increased serum creatinine in STNx rats was not significantly affected by apocynin. Data were expressed as mean±SD. n=10 for each group; *P<0.01 vs Sham group; #P<0.01 vs Apocynin+STNx group. DBP indicates diastolic blood pressure; SBP, systolic blood pressure; STNx, 5/6 subtotally nephrectomized.
Table 3.
Survival Rate, Plasma Ang II, and Echocardiographic Parameters in Rats at Week 8 After Surgery
| Sham Operated(n=10) | STNx(n=10) | STNx+Apocynin(n=10) | |
|---|---|---|---|
| Survival rate | 10/10 | 10/13 | 10/12 |
| Ang II, ng/L | 281 (152, 359) | 412 (251, 576)* | 320 (172, 441)† |
| Body weight, g | 360±24 | 259±20* | 290±23*† |
| LV weight, g | 0.59±0.05 | 0.82±0.05* | 0.65±0.03*† |
| LV/body weight, g/kg | 1.70±0.13 | 3.20±0.21* | 2.14±0.16*† |
| LVPWd, mm | 1.13±0.03 | 1.36±0.09* | 1.25±0.07*† |
| LVEDD, mm | 7.48±0.49 | 7.685±0.62 | 7.49±0.43 |
| LVESD, mm | 4.87±0.33 | 5.98±0.35* | 5.07±0.24† |
| FS% | 32.2±1.3 | 21.9±2.7* | 28.7±2.0*† |
| EF% | 65.1±2.4 | 49.2±4.7* | 61.1±4.4† |
Ang indicates angiotensin II; STNx, 5/6 subtotally nephrectomized; LV, left ventricular; LVPWd, left ventricular posterior wall thickness at diastole; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; FS, fraction shortening; EF, ejection fraction.
P<0.01 vs sham-operated group.
P<0.01 vs STNx group.
STNx rats showed a significant decrease in body weight (Table3) but a remarked increase in left ventricular weight compared with sham-operated rats. STNx resulted in a significant increase in the ratio of left ventricular weight to body weight compared with sham surgery (Table3), indicating significantly cardiac remodeling. In addition, echocardiographic examination showed a significant increase in LVPWd in STNx rats compared with sham-operated rats. Increased LVESD and decreased left ventricular fractional shortening and EF were observed in STNx rats (Table3). Masson staining revealed increased cardiac interstitial fibrosis in STNx rats (Figure2A and 2B). The expression of FGF-2 was upregulated in STNx rats (Figure2C). These findings demonstrated that STNx rats with impaired renal function showed remarkable cardiac impairments, including cardiac remodeling, interstitial fibrosis, and cardiac dysfunction, all of which were improved with apocynin treatment. Increased circulating Ang II was found in STNx rats. Also, STNx rats showed increased cardiac oxidative stress compared with sham-operated rats, as indicated by elevated levels of MDA in left ventricular tissue (3.86±0.68 versus 2.05±0.23 nmol/mg protein, P<0.01), which was also attenuated by apocynin (3.86±0.68 versus 2.32±0.18 nmol/mg protein, P<0.01) (Figure2D). Apocynin markedly reduced the increased NOX activity in STNx rats (Figure2E). These findings indicated that apocynin attenuated cardiac remodeling, interstitial fibrosis, and cardiac dysfunction in rats with impaired renal function via inhibiting oxidative stress.
Figure 2.
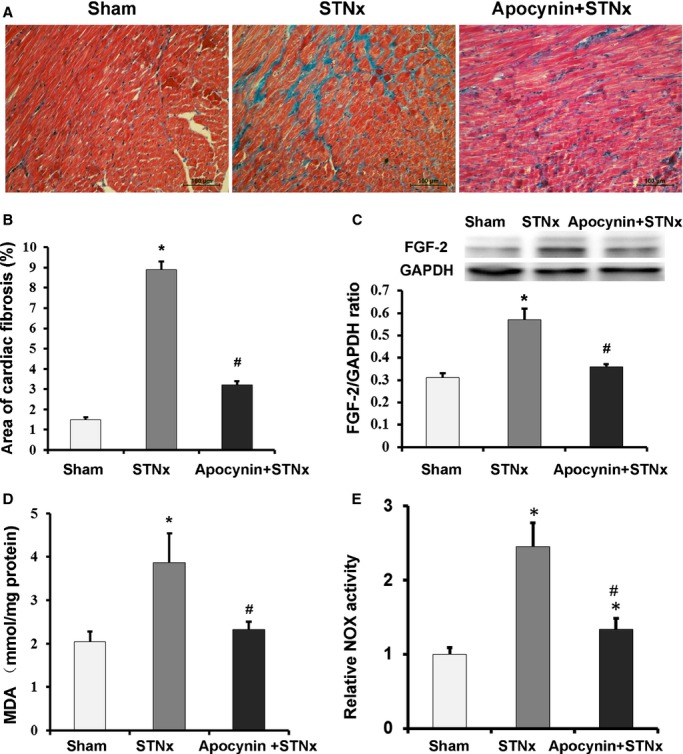
Apocynin attenuated cardiac fibrosis, expression of fibroblast growth factor-2, malondialdehyde and NOX activity in STNx rats. A, Representative Masson staining micrographs show that the increased cardiac interstitial fibrosis in STNx rats was attenuated by apocynin (bar=200 μm). B, Bar graph shows the interstitial fibrosis volume fractions (n=40 fields). C, The expression level of cardiac FGF-2 (n=6). D, MDA levels in left ventricular tissue homogenates (n=6). E, NOX activity was measured with lucigenin-enhanced chemiluminescence assay. The NOX activities in different groups were normalized to controls (n=4). Data were expressed as mean±SD. *P<0.01 vs Sham group; #P<0.01 vs STNx group. FGF-2 indicates fibroblast growth factor-2; GAPDH, anti–glyceraldehyde 3-phosphate dehydrogenase; MDA, malondialdehyde; NOX, nicotinamide adenine dinucleotide phosphate oxidase; STNx, 5/6 subtotally nephrectomized.
Apocynin Reduced NOX-Dependent Oxidative Stress and Inhibited Expressions of FGF-2 and Fibrosis Biomarkers in Cardiac Fibroblasts Treated With Ang II
To explore whether the cardioprotective effects of apocynin in type 4 CRS are independent of its antihypertensive effect, we determined the effect of apocynin on cardiac fibroblasts treated with Ang II in vitro. Ang II was widely used as a stimulator for oxidative stress due to its important role in CKD. Western blot analysis showed that Ang II upregulated the expressions of FGF-2 and fibrosis biomarkers, including procollagen I, procollagen III, and TGF-β in cardiac fibroblasts (Figure3). Ang II also induced a significant increase in generally intracellular ROS (Figure4A) and NOX activity (Figure5) in cardiac fibroblasts, indicating increased oxidative stress. The increases in general and NOX-dependent superoxide anion and upregulations of FGF-2 and fibrosis biomarkers induced by Ang II were attenuated with apocynin treatment. However, apocynin had no significant effect on Ang II–induced mitochondrial superoxide anion production (Figure4B). Therefore, these in vitro findings demonstrated that apocynin had an antifibrotic effect on Ang II–treated cardiac fibroblasts through its inhibition of NOX-dependent oxidative stress pathway, independent of its antihypertensive effect.
Figure 3.
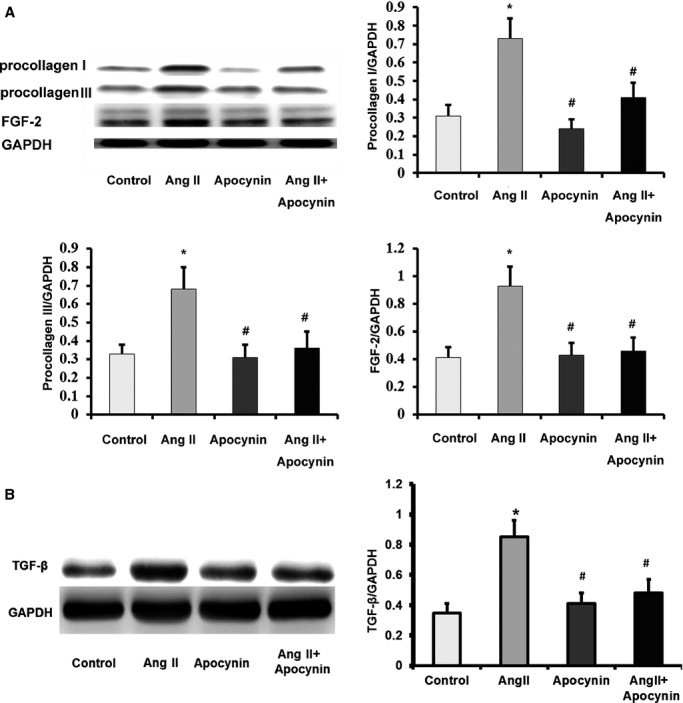
Apocynin attenuated Ang II–induced upregulations of fibrosis biomarkers and fibroblast growth factor-2 in cardiac fibroblasts. A, Western blot results showed that Ang II–induced upregulations of procollagen I, procollagen III and FGF-2 were reduced by apocynin (n=4). B, Apocynin attenuated Ang II–induced upregulation of TGF-β (n=4). Data were expressed as mean±SD. *P<0.01 vs Control group; #P<0.01 vs Ang II group. Ang II indicates angiotensin II; FGF-2, fibroblast growth factor-2; GAPDH, anti–glyceraldehyde 3-phosphate dehydrogenase; TGF-β, transforming growth factor-beta.
Figure 4.
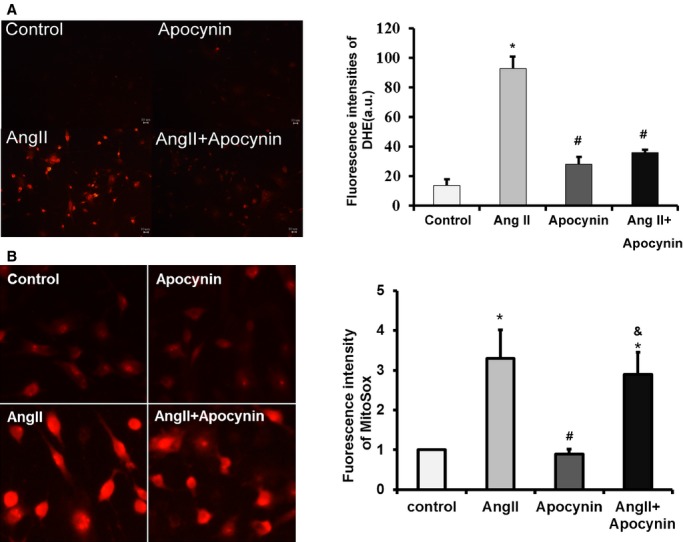
Apocynin reduced intracellular superoxide anion, but had no significant effect on mitochondrial superoxide anion production. A, Representative fluorescence micrographs (×200) on detecting intracellular superoxide anion with DHE. The bar graph shows the fluorescence intensities of DHE measured wtih a multimode microplate reader (n=3). B, Representative fluorescence micrographs (×400) on detecting mitochondrial superoxide anion with MitoSOX Red. The bar graph shows the relative fluorescence intensities normalized to controls (n=3). Data were expressed as mean±SD. *P<0.01 vs Control group; #P<0.01 vs Ang II group; &P<0.01 vs Apocynin group. Ang II indicates angiotensin II; DHE, dihydroethidium.
Figure 5.
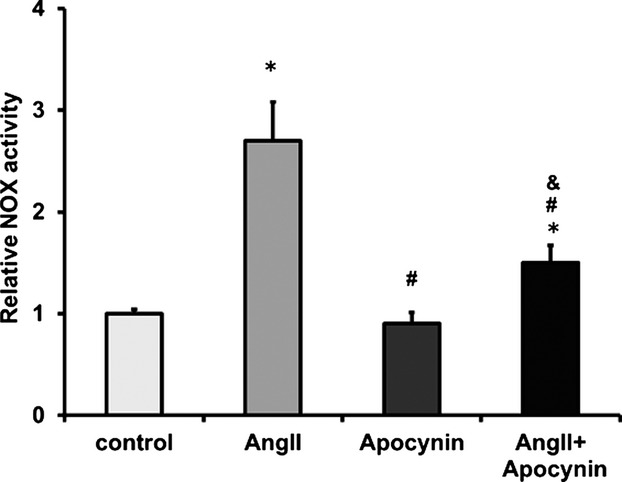
Apocynin inhibited NOX activity in Ang II–treated cardiac fibroblasts. NOX activity was measured with lucigenin-enhanced chemiluminescence assay. The NOX activities in different groups were normalized to controls (n=3). Data were expressed as mean±SD. *P<0.01 vs Control group; #P<0.01 vs Ang II group; &P<0.01 vs Apocynin group. Ang II indicates angiotensin II; NOX, nicotinamide adenine dinucleotide phosphate oxidase.
Beneficial Effect of Apocynin Occurred Through Inhibition of ERK1/2 Activation and Subsequent FGF-2 Expression
The ERK1/2 pathway was activated in STNx rats, which was inhibited by apocynin treatment (Figure6). In the in vitro study, Ang II induced phosphorylation of ERK1/2 in cardiac fibroblasts, while pretreatment with apocynin showed an inhibitory effect on ERK1/2 phosphorylation (Figure7A). Furthermore, ERK1/2 inhibition with U0126 suppressed Ang II–induced upregulations of FGF-2, procollagen I, procollagen III, and TGF-β in cardiac fibroblasts (Figure7B and 7C). These results demonstrated that the protective effect of apocynin against Ang II–induced fibrosis occurred via reducing NOX-dependent oxidative stress and inhibition of subsequent ERK1/2 activation and expression of FGF-2.
Figure 6.
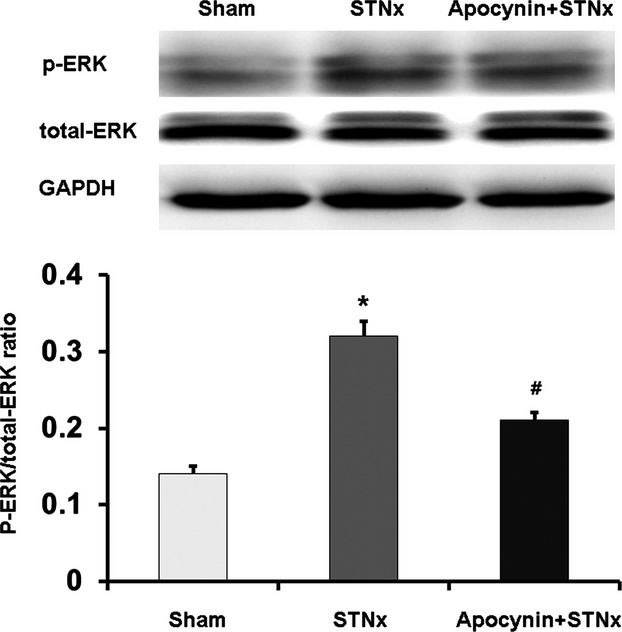
Apocynin inhibited the phosphorylation of ERK1/2 in STNx rats. Data were expressed as mean±SD. *P<0.01 vs Control group; #P<0.01 vs Ang II group. Ang II indicates angiotensin II; ERK1/2, extracellular signal-regulated kinase 1/2; GAPDH, anti–glyceraldehyde 3-phosphate dehydrogenase; p-ERK, phosphorylation extracellular signal-regulated kinase; STNx, 5/6 subtotally nephrectomized.
Figure 7.
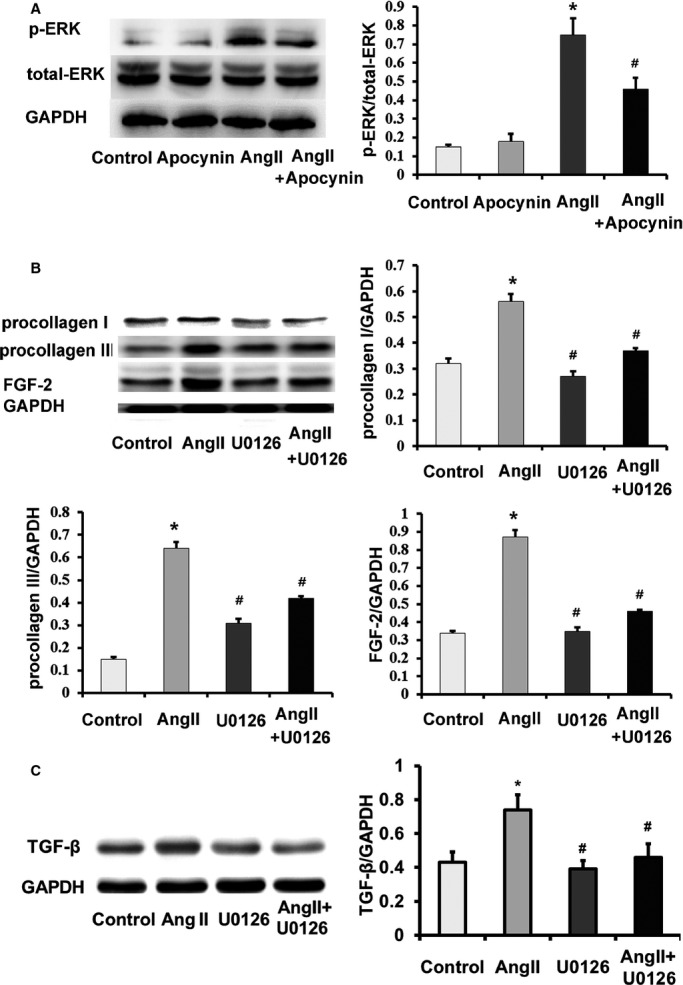
Apocynin attenuated cardiac fibrosis via inhibiting extracellular signal-regulated kinase 1/2 activation and fibroblast growth factor-2 expression. A, Ang II–induced ERK1/2 p-ERK was inhibited by apocynin. B and C, Ang II–induced expressions of FGF-2 and fibrosis biomarkers, including procollagen I procollagen III and TGF-β, were reduced by U0126 (ERK1/2 inhibitor) in cardiac fibroblasts. Data were expressed as mean±SD; n=3 for each group. *P<0.01 vs Control group; #P<0.01 vs Ang II group. Ang II indicates angiotensin II; p-ERK, phosphorylation extracellular signal-regulated kinase; FGF-2, fibroblast growth factor-2; TGF-β, transforming growth factor-beta; GAPDH, anti–glyceraldehyde 3-phosphate dehydrogenase; ERK1/2, extracellular signal-regulated kinase 1/2.
Discussion
Growing attention has been paid to type 4 CRS due to its high morbidity and mortality. We found that oxidative stress was involved in the development of type 4 CRS. In patients with type 4 CRS, oxidative stress was remarkably elevated and significantly associated with cardiac remodeling and dysfunction. Furthermore, the animal study, for the first time, demonstrated that apocynin effectively attenuated cardiac remodeling and interstitial fibrosis and improved cardiac function in a rat model of type 4 CRS through inhibition of oxidative stress and FGF-2 expression. Our in vitro results indicated that the protective effects of apocynin were partly mediated by inhibition of NOX-dependent oxidative stress-activated ERK1/2 pathway and FGF-2 upregulation and independent of hemodynamic changes.
The pathophysiological mechanism linking renal and cardiac impairments in type 4 CRS is not fully understood. The pivotal role of oxidative stress in the pathogenesis of cardiovascular diseases, including hypertension, cardiac remodeling, and heart failure, has long been emphasized.25 Previous studies indicated that increased oxidative stress also played a pivotal role in CSR.26–28 In our cross-sectional study, SOD was significantly decreased and related to cardiac remodeling and dysfunction in patients with type 4 CRS. Furthermore, increased oxidative stress was detected in STNx rats with remarkable cardiac impairments. Although there is not a well-accepted model of type 4 CRS so far, the cardiac impairments in STNx rats, including cardiac remodeling, interstitial fibrosis, and cardiac dysfunction, mimicked the cardiac changes in this syndrome.1
NOX is a major source of ROS within the heart.29 The expression and activity of NOX are upregulated in clinical and experimental cardiovascular diseases.30 NOX activity also increased significantly in STNx rats and Ang II–induced cardiac fibroblasts in the present study. Experimental studies find that inhibition of NOX have cardioprotective effects, including reducing blood pressure, inhibiting cardiac remodeling, and improving cardiac function.30 Inhibition of oxidative stress with antioxidants, including NOX inhibitor, may also be a potential therapeutic strategy for CRS.26–28 However, related studies assessing antioxidant treatments in type 4 CRS are rare. A small-sample randomized controlled trial by Camuglia et al31 found that antioxidant treatment with N-acetylcysteine improves forearm blood flow in patients with CRS (n=9). This clinical trial indicated the beneficial effect of antioxidant treatment in CRS. In the present study, inhibition of NOX with apocynin ameliorated the increased oxidative stress and accompanied cardiac impairments in STNx rats. The present experimental study adds evidence to the beneficial effects of antioxidant treatment with apocynin in type 4 CRS. However, we should notice the gaps between experimental studies and clinical trials concerning antioxidant treatments in cardiovascular diseases. Although increasing experimental studies discover the beneficial effects of antioxidant treatments on cardiovascular diseases, the results of clinical trials so far are controversial. Vitamins are the most commonly used antioxidants in clinical trials. Recently, a meta-analysis found that antioxidant vitamins, including vitamin C, vitamin E, and beta-carotene, had no significant effect on major cardiovascular diseases.32 The differences in antioxidants and dosages may influence their curative effects on cardiovascular diseases. Some antioxidants show dose-dependent antioxidative and prooxidative properties.33 In addition, low to moderate ROS levels promote endogenous antioxidant response by upregulating antioxidant enzymes.34,35 Therefore, the regulation of oxidative stress level is complicated, and there is a long way to go to find clinically effective antioxidants and related therapeutic regimens. In total, our findings confirmed the cardioprotective effects of apocynin in type 4 CRS.
Apart from the cardioprotective effects, apocynin showed an antihypertensive effect. This was accordance with previous studies in other hypertension models.36 To investigate whether the cardioprotective effects of apocynin were independent of its antihypertensive effect, we conducted an in vitro experiment in cardiac fibroblasts, using Ang II as a stimulator due to its significant effect in CKD.37 In vitro results found a significant inhibitory effect of apocynin on fibrosis and NOX-dependent oxidative stress in cardiac fibroblasts. Although apocynin had an antihypertensive effect, previous studies found that lowering blood pressure alone could not improve CKD-induced cardiac fibrosis.38,39 Therefore, these results indicated that the effect of apocynin on cardiac interstitial fibrosis may be independent of its antihypertensive effect.
Studies demonstrate that apcynin reduces NOX activity by inhibiting the expressions of NOX subunits and translocation from cytosol to the membrane.40,41 The cardioprotective effects of apocynin occurred via attenuation of NOX-dependent oxidative stress, but the potential downstream mechanisms are not clear.14,42 Li et al found that osteopontin might be involved in the protection of apocynin against cardiac fibrosis.14 Liu et al found that upregulating the expression and activity of SERCA2α was associated with the beneficial effect of apocynin on cardiac dysfunction.42 The present study found that the phosphorylation of ERK1/2 and overexpressions of fibrosis biomarkers in both STNx rats and cardiac fibroblasts treated with Ang II were markedly suppressed by apocynin. Furthermore, ERK1/2 inhibition suppressed the profibrotic effects of Ang II on cardiac fibroblasts. These results revealed that apocynin-mediated suppression of cardiac fibrosis was partly through inhibiting NOX-dependent oxidative stress-activated ERK1/2 pathway.
FGF-2 is an important profibrotic factor. Findings from our previous study18 and others’19 revealed that FGF-2 played a pivotal role in cardiac remodeling and fibrosis and that cardiac nonmyocytes like fibroblasts were the main sources of cardiac FGF-2. In the present study, we found that FGF-2 was also involved in cardiac impairments in type 4 CRS. Many factors that are believed to induce oxidative stress, including Ang II, endothelin-1, and transforming growth factor-β1 can upregulate FGF-2 and are suppressed by antioxidants.43,44 In our study, the upregulated FGF-2 and increased oxidative stress in STNx rats and Ang II–treated cardiac fibroblasts were inhibited by apocynin. Therefore, the harmful effects of oxidative stress on cardiac tissue in type 4 CRS may occur partly through upregulation of FGF-2. Previous studies found that FGF-2 activated the ERK1/2 pathway in fibroblast and endothelial cells.45,46 However, we found that ERK1/2 inhibitor attenuated the upregulation of FGF-2 in fibroblasts. These findings indicated that there may be a positive feedback mechanism between FGF-2 and ERK1/2 pathway, but further investigations are required. In all, our results demonstrated that the cardioprotective effects of apocynin were due to reducing NOX-dependent oxidative stress and possibly inhibition of the positive feedback mechanism between FGF-2 and ERK1/2. Therefore, NOX-dependent oxidative stress–ERK1/2-FGF-2 may be a novel mechanism involved in the cardioprotective effects of apocynin.
In addition, studies found that apocynin had protective effects on kidneys in some models of kidney disease, including attenuating selective albuminuria, tubular apoptosis, and interstitial fibrosis.47,48 However, in the present study, apocynin showed no significant effect on serum creatinine in STNx rats. The possible explanation may be due to the difference in animal models. An irreversible reduction in renal mass and function after STNx surgery may be hard to improve with apocynin treatment.
There are some limitations in the present study. First, the study exploring the relationship between oxidative stress and cardiac impairments in patients with type 4 CRS was a cross-sectional study with a small sample size. Second, in this animal study, we did not use RAAS blockers as positive control treatment. Previous studies have already demonstrated that RAAS blockers could attenuate CKD-related oxidative stress and cardiac remodeling. We will compare the different as well as synergistic effects of apocynin and RAAS blockers on type 4 CRS in future studies. Also, although apocynin is a commonly used antioxidant, there are some controversies about its specificity.49 However, in this study, we did find a significant effect of apocynin on reducing oxidative stress. Future studies need to evaluate the effects of other, more-selective antioxidants in type 4 CRS.
Conclusion
Oxidative stress plays a pivotal role in cardiac injury accompanied by CKD. Increased oxidative stress was significantly linked to the cardiac remodeling and dysfunction in patients with type 4 CRS. Our results showed, for the first time, that apocynin attenuated cardiac injury in type 4 CRS. The mechanism may be through inhibition of NOX-dependent oxidative stress-activated ERK1/2 pathway and subsequent FGF-2 upregulation. The present study added evidence to the cardioprotective effects of antioxidant treatment and underlined the involvement of FGF-2 in type 4 CRS. Given the high morbidity and mortality of this syndrome, and the controversy on antioxidant treatment in cardiovascular diseases, more studies are needed to assess the roles of oxidative stress and the effect of different antioxidants in type 4 CRS as well as other cardiovascular diseases.
Sources of Funding
This work was supported in part by NSFC (81422011, 81370837, and 81170647 to Dr Huang), Natural Science Foundation of Guangdong Province (2014A030313035), The Science Foundation of China Society of Integrated Traditional Chinese and Western Medicine-Shanghai Hutchison Pharmaceuticals Limited (2013002), and a Discovery Cardiovascular Research grant (DFCMFDA201238).
Disclosures
None.
References
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- Tumlin JA, Costanzo MR, Chawla LS, Herzog CA, Kellum JA, McCullough PA, Ronco C. Cardiorenal syndrome type 4: insights on clinical presentation and pathophysiology from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:158–173. doi: 10.1159/000349972. [DOI] [PubMed] [Google Scholar]
- Bello AK, Nwankwo E, El Nahas AM. Prevention of chronic kidney disease: a global challenge. Kidney Int Suppl. 2005;98:S11–S17. doi: 10.1111/j.1523-1755.2005.09802.x. [DOI] [PubMed] [Google Scholar]
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- Clementi A, Virzi GM, Goh CY, Cruz DN, Granata A, Vescovo G, Ronco C. Cardiorenal syndrome type 4: a review. Cardiorenal Med. 2013;3:63–70. doi: 10.1159/000350397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi A, Virzi GM, Brocca A, de Cal M, Vescovo G, Granata A, Ronco C. Cardiorenal syndrome type 4: management. Blood Purif. 2013;36:200–209. doi: 10.1159/000356369. [DOI] [PubMed] [Google Scholar]
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- Lekawanvijit S, Kompa AR, Wang BH, Kelly DJ, Krum H. Cardiorenal syndrome: the emerging role of protein-bound uremic toxins. Circ Res. 2012;111:1470–1483. doi: 10.1161/CIRCRESAHA.112.278457. [DOI] [PubMed] [Google Scholar]
- Tucker PS, Dalbo VJ, Han T, Kingsley MI. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers. 2013;18:103–115. doi: 10.3109/1354750X.2012.749302. [DOI] [PubMed] [Google Scholar]
- Zhou LL, Cao W, Xie C, Tian J, Zhou Z, Zhou Q, Zhu P, Li A, Liu Y, Miyata T, Hou FF, Nie J. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82:759–770. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]
- Burrell LM, Burchill L, Dean RG, Griggs K, Patel SK, Velkoska E. Chronic kidney disease: cardiac and renal angiotensin-converting enzyme (ACE) 2 expression in rats after subtotal nephrectomy and the effect of ACE inhibition. Exp Physiol. 2012;97:477–485. doi: 10.1113/expphysiol.2011.063156. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Campbell RC, Warnock DG. Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Semin Nephrol. 2004;24:101–114. doi: 10.1016/j.semnephrol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Petronio MS, Zeraik ML, Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18:2821–2839. doi: 10.3390/molecules18032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Li XB, Guo SJ, Chu SL, Gao PJ, Zhu DL, Niu WQ, Jia N. Apocynin attenuates oxidative stress and cardiac fibrosis in angiotensin II-induced cardiac diastolic dysfunction in mice. Acta Pharmacol Sin. 2013;34:352–359. doi: 10.1038/aps.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Chen SS, Chen Y, Ahokas RA, Sun Y. Kidney fibrosis in hypertensive rats: role of oxidative stress. Am J Nephrol. 2008;28:548–554. doi: 10.1159/000115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SY, Lin IH, Shieh TM, Ko HA, Chen HI, Chi TC, Chang SS, Hsu YC. Cell hypertrophy and MEK/ERK phosphorylation are regulated by glyceraldehyde-derived AGEs in cardiomyocyte H9c2 cells. Cell Biochem Biophys. 2013;66:537–544. doi: 10.1007/s12013-012-9501-8. [DOI] [PubMed] [Google Scholar]
- Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, Ogawa S. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;89:661–669. doi: 10.1161/hh2001.098873. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang T, Zhang K, Liu Y, Huang F, Zhu X, Wang MH, Tang W, Wang J, Huang H. Deletion of soluble epoxide hydrolase attenuates cardiac hypertrophy via down-regulation of cardiac fibroblasts-derived fibroblast growth factor-2. Crit Care Med. 2014;42:e345–e354. doi: 10.1097/CCM.0000000000000226. [DOI] [PubMed] [Google Scholar]
- Kardami E, Jiang ZS, Jimenez SK, Hirst CJ, Sheikh F, Zahradka P, Cattini PA. Fibroblast growth factor 2 isoforms and cardiac hypertrophy. Cardiovasc Res. 2004;63:458–466. doi: 10.1016/j.cardiores.2004.04.024. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- Renke M, Tylicki L, Knap N, Rutkowski P, Neuwelt A, Petranyuk A, Larczynski W, Wozniak M, Rutkowski B. High-dose angiotensin-converting enzyme inhibitor attenuates oxidative stress in patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24:689–690. doi: 10.1093/ndt/gfn665. [DOI] [PubMed] [Google Scholar]
- Linz P, Amann K, Freisinger W, Ditting T, Hilgers KF, Veelken R. Sensory neurons with afferents from hind limbs: enhanced sensitivity in secondary hypertension. Hypertension. 2006;47:527–531. doi: 10.1161/01.HYP.0000199984.78039.36. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chan MM, Andrews MC, Mori TA, Croft KD, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am J Hypertens. 2005;18:910–916. doi: 10.1016/j.amjhyper.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Husain K, Ferder L, Mizobuchi M, Finch J, Slatopolsky E. Combination therapy with paricalcitol and enalapril ameliorates cardiac oxidative injury in uremic rats. Am J Nephrol. 2009;29:465–472. doi: 10.1159/000178251. [DOI] [PubMed] [Google Scholar]
- Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.03.005. pii: S1537-1891(15)00042-7. doi: 10.1016/j.vph.2015.03.005 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Morena M, Delbosc S, Dupuy AM, Canaud B, Cristol JP. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. Hemodial Int. 2005;9:37–46. doi: 10.1111/j.1492-7535.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- Rubattu S, Mennuni S, Testa M, Mennuni M, Pierelli G, Pagliaro B, Gabriele E, Coluccia R, Autore C, Volpe M. Pathogenesis of chronic cardiorenal syndrome: is there a role for oxidative stress? Int J Mol Sci. 2013;14:23011–23032. doi: 10.3390/ijms141123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep. 2012;14:360–365. doi: 10.1007/s11906-012-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camuglia AC, Maeder MT, Starr J, Farrington C, Kaye DM. Impact of N-acetylcysteine on endothelial function, B-type natriuretic peptide and renal function in patients with the cardiorenal syndrome: a pilot cross over randomised controlled trial. Heart Lung Circ. 2013;22:256–259. doi: 10.1016/j.hlc.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e56803. doi: 10.1371/journal.pone.0056803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadino AM, Cossu A, Giordo R, Zinellu A, Sotgia S, Vardeu A, Hoa PT, le Nguyen HV, Carru C, Pintus G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem Toxicol. 2015;78:10–16. doi: 10.1016/j.fct.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Korantzopoulos P, Cereceda M, Asenjo R, Zamorano J, Villalabeitia E, Baeza C, Aguayo R, Castillo R, Carrasco R, Gormaz JG. A randomized controlled trial to prevent post-operative atrial fibrillation by antioxidant reinforcement. J Am Coll Cardiol. 2013;62:1457–1465. doi: 10.1016/j.jacc.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Tain YL, Hsu CN, Huang LT, Lau YT. Apocynin attenuates oxidative stress and hypertension in young spontaneously hypertensive rats independent of ADMA/NO pathway. Free Radic Res. 2012;46:68–76. doi: 10.3109/10715762.2011.639069. [DOI] [PubMed] [Google Scholar]
- Turner JM, Bauer C, Abramowitz MK, Melamed ML, Hostetter TH. Treatment of chronic kidney disease. Kidney Int. 2012;81:351–362. doi: 10.1038/ki.2011.380. [DOI] [PubMed] [Google Scholar]
- Amann K, Tyralla K, Gross ML, Schwarz U, Tornig J, Haas CS, Ritz E, Mall G. Cardiomyocyte loss in experimental renal failure: prevention by ramipril. Kidney Int. 2003;63:1708–1713. doi: 10.1046/j.1523-1755.2003.00927.x. [DOI] [PubMed] [Google Scholar]
- Tyralla K, Adamczak M, Benz K, Campean V, Gross ML, Hilgers KF, Ritz E, Amann K. High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. PLoS One. 2011;6:e15287. doi: 10.1371/journal.pone.0015287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys. 2007;457:134–141. doi: 10.1016/j.abb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, Xia W, Tang Y, Li H, Huang C. NADPH oxidase inhibition ameliorates cardiac dysfunction in rabbits with heart failure. Mol Cell Biochem. 2010;343:143–153. doi: 10.1007/s11010-010-0508-4. [DOI] [PubMed] [Google Scholar]
- Black SM, DeVol JM, Wedgwood S. Regulation of fibroblast growth factor-2 expression in pulmonary arterial smooth muscle cells involves increased reactive oxygen species generation. Am J Physiol Cell Physiol. 2008;294:C345–C354. doi: 10.1152/ajpcell.00216.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago JJ, Ma X, McNaughton LJ, Nickel BE, Bestvater BP, Yu L, Fandrich RR, Netticadan T, Kardami E. Preferential accumulation and export of high molecular weight FGF-2 by rat cardiac non-myocytes. Cardiovasc Res. 2011;89:139–147. doi: 10.1093/cvr/cvq261. [DOI] [PubMed] [Google Scholar]
- Koyama H, Olson NE, Dastvan FF, Reidy MA. Cell replication in the arterial wall: activation of signaling pathway following in vivo injury. Circ Res. 1998;82:713–721. doi: 10.1161/01.res.82.6.713. [DOI] [PubMed] [Google Scholar]
- Xiao L, Du Y, Shen Y, He Y, Zhao H, Li Z. TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front Biosci (Landmark Ed) 2012;17:2667–2674. doi: 10.2741/4077. [DOI] [PubMed] [Google Scholar]
- Kinugasa S, Tojo A, Sakai T, Tsumura H, Takahashi M, Hirata Y, Fujita T. Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase. Kidney Int. 2011;80:1328–1338. doi: 10.1038/ki.2011.282. [DOI] [PubMed] [Google Scholar]
- Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int. 2009;75:156–166. doi: 10.1038/ki.2008.509. [DOI] [PubMed] [Google Scholar]
- Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


