Figure 1.
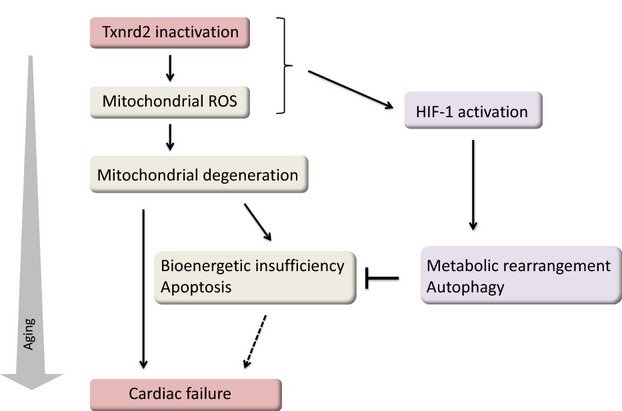
A possible regulatory mechanism by which Txnrd2 inactivation leads to the progression of age-related heart failure. A prominent hypothesis of Txnrd2 inactivation postulates that accumulating mitochondrial damage by increased generation of ROS results in progressive mitochondrial degeneration with consequent bioenergic insufficiency and cardiomyocyte death during aging. Txnrd2 inactivation induces stabilization of the α subunit of HIF-1 and transcriptional activation of HIF-1. The HIF-1–driven metabolic adaptation and increased autophagy may be necessary for resistance to mitochondrial oxidative damage and an energy crisis in the aged cardiomyocytes. These pathways suggest integrated regulation of the mitochondrial thioredoxin system that confers resistance to oxidative stress in cardiac senescence. HIF-1 indicates hypoxia-inducible factor 1; ROS, reactive oxygen species; Txnrd2, thioredoxin reductase 2.
