Abstract
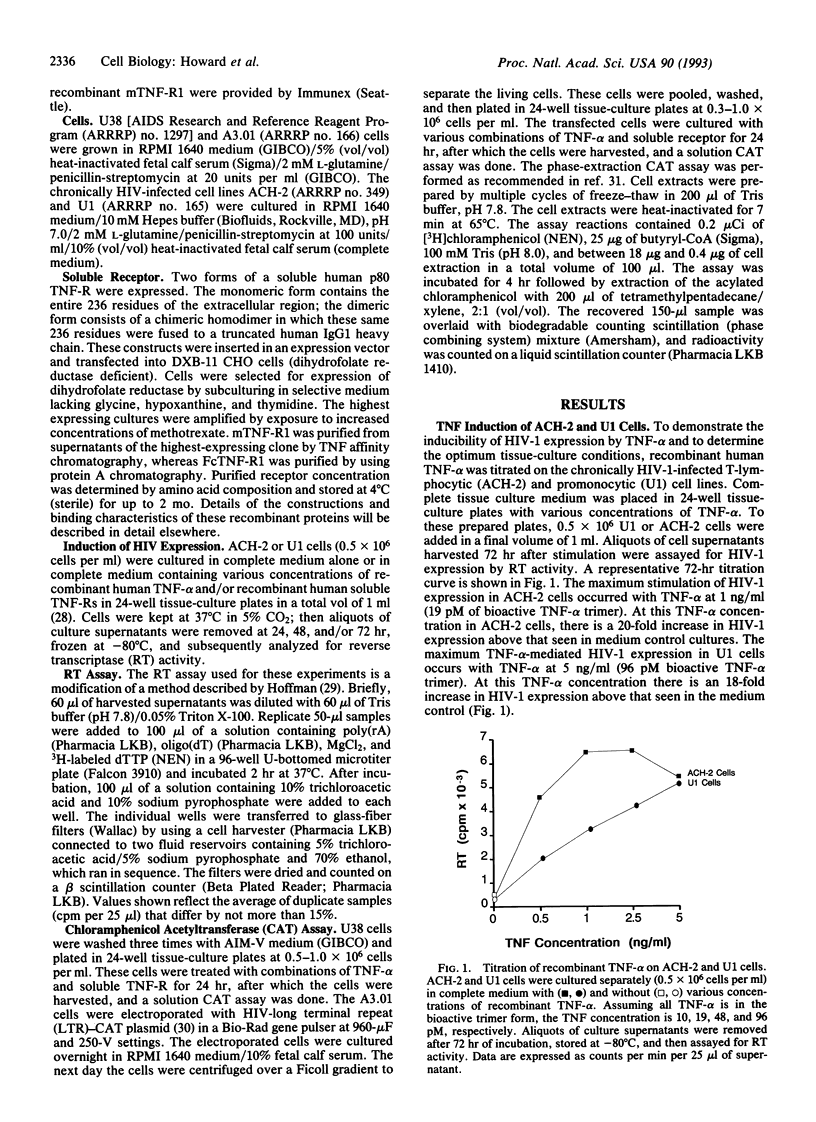
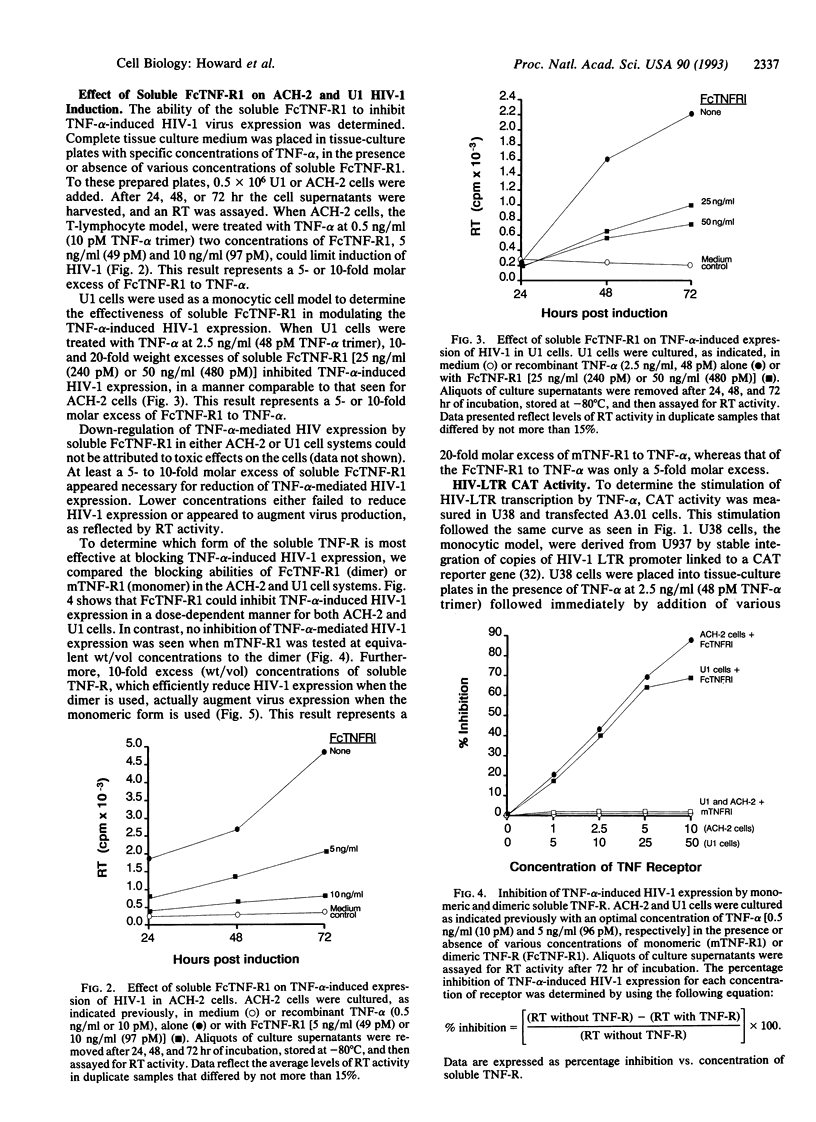
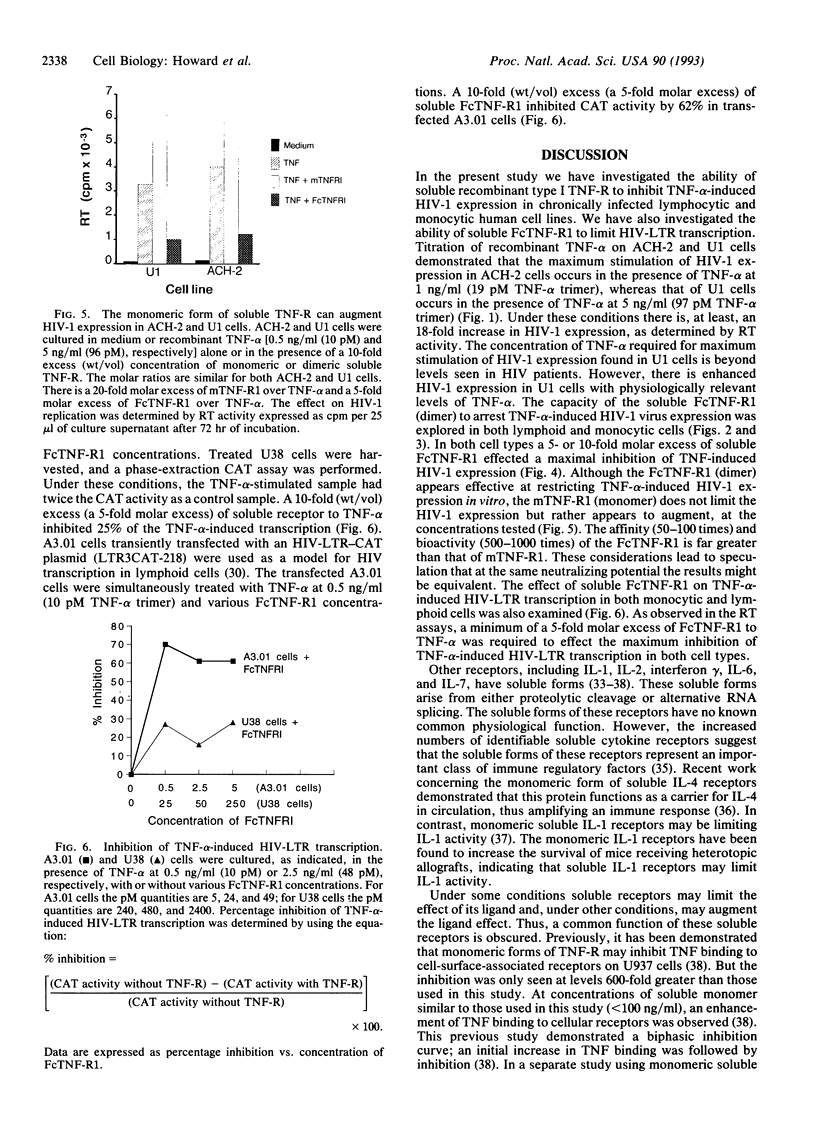
The inflammatory cytokine tumor necrosis factor alpha (TNF-alpha) has been shown to stimulate human immunodeficiency virus type 1 (HIV-1) replication in both chronically and acutely infected T lymphocytes and monocytes. Transcriptional activation of the HIV long terminal repeat and subsequent increase in virus production are linked to TNF activation of the cellular transcription factor NF-kappa B. Here we report the use of two forms of soluble recombinant type 1 (p80) TNF receptor to inhibit TNF-induced HIV activation in vitro. One receptor form is a monomer containing the entire 236 residues of the extracellular (ligand-binding) portion of p80. A second receptor form is a chimeric homodimer containing these residues fused to a truncated human IgG1 immunoglobulin heavy chain and, thus, resembles a bivalent antibody without light chains. These recombinant receptor proteins were tested for their ability to inhibit TNF-alpha-induced expression of HIV-1 in chronically infected human cell lines. We also examined the ability of the soluble receptors to limit the activation of the HIV-long terminal repeat transcription. The soluble TNF receptor dimer was most effective at blocking TNF-alpha-induced HIV-1 expression in both monocytoid and lymphoid cells. The molar ratio of TNF-receptor dimer to TNF-alpha found to be most effective was, at least, 5:1. We conclude that at specific TNF/soluble TNF-receptor dimer ratios, TNF-alpha-induced HIV-1 transcription and expression can be limited in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Engelmann H., Maor Y., Brakebusch C., Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992 Feb 1;175(2):323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderka D., Englemann H., Hornik V., Skornick Y., Levo Y., Wallach D., Kushtai G. Increased serum levels of soluble receptors for tumor necrosis factor in cancer patients. Cancer Res. 1991 Oct 15;51(20):5602–5607. [PubMed] [Google Scholar]
- Arakawa T., Yphantis D. A. Molecular weight of recombinant human tumor necrosis factor-alpha. J Biol Chem. 1987 Jun 5;262(16):7484–7485. [PubMed] [Google Scholar]
- Camussi G., Albano E., Tetta C., Bussolino F. The molecular action of tumor necrosis factor-alpha. Eur J Biochem. 1991 Nov 15;202(1):3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- Clouse K. A., Cosentino L. M., Weih K. A., Pyle S. W., Robbins P. B., Hochstein H. D., Natarajan V., Farrar W. L. The HIV-1 gp120 envelope protein has the intrinsic capacity to stimulate monokine secretion. J Immunol. 1991 Nov 1;147(9):2892–2901. [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann H., Aderka D., Rubinstein M., Rotman D., Wallach D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J Biol Chem. 1989 Jul 15;264(20):11974–11980. [PubMed] [Google Scholar]
- Fanslow W. C., Sims J. E., Sassenfeld H., Morrissey P. J., Gillis S., Dower S. K., Widmer M. B. Regulation of alloreactivity in vivo by a soluble form of the interleukin-1 receptor. Science. 1990 May 11;248(4956):739–742. doi: 10.1126/science.2139736. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Vitetta E. S. Evidence that natural murine soluble interleukin 4 receptors may act as transport proteins. J Exp Med. 1991 Sep 1;174(3):673–681. doi: 10.1084/jem.174.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Wu F. K., Mitsuyasu R., Gaynor R. B. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987 Dec 1;6(12):3761–3770. doi: 10.1002/j.1460-2075.1987.tb02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Barrett K., Chantry D., Turner M., Feldmann M. Cloning of human tumor necrosis factor (TNF) receptor cDNA and expression of recombinant soluble TNF-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7380–7384. doi: 10.1073/pnas.87.19.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Aggarwal B. B. Inhibition of ligand binding and antiproliferative effects of tumor necrosis factor and lymphotoxin by soluble forms of recombinant P60 and P80 receptors. Biochem Biophys Res Commun. 1992 Jan 31;182(2):638–643. doi: 10.1016/0006-291x(92)91780-t. [DOI] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Jacobs C. A., Lynch D. H., Roux E. R., Miller R., Davis B., Widmer M. B., Wignall J., VandenBos T., Park L. S., Beckmann M. P. Characterization and pharmacokinetic parameters of recombinant soluble interleukin-4 receptor. Blood. 1991 Jun 1;77(11):2396–2403. [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Koyanagi Y., O'Brien W. A., Zhao J. Q., Golde D. W., Gasson J. C., Chen I. S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988 Sep 23;241(4873):1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Remick D. G., Strieter R. M., Larrick J. W. Mechanisms that regulate the production and effects of tumor necrosis factor-alpha. Crit Rev Immunol. 1989;9(2):93–117. [PubMed] [Google Scholar]
- Latham P. S., Lewis A. M., Varesio L., Pavlakis G. N., Felber B. K., Ruscetti F. W., Young H. A. Expression of human immunodeficiency virus long terminal repeat in the human promonocyte cell line U937: effect of endotoxin and cytokines. Cell Immunol. 1990 Sep;129(2):513–518. doi: 10.1016/0008-8749(90)90225-g. [DOI] [PubMed] [Google Scholar]
- Lau A. S., Der S. D., Read S. E., Williams B. R. Regulation of tumor necrosis factor receptor expression by acid-labile interferon-alpha from AIDS sera. AIDS Res Hum Retroviruses. 1991 Jun;7(6):545–552. doi: 10.1089/aid.1991.7.545. [DOI] [PubMed] [Google Scholar]
- Lesslauer W., Tabuchi H., Gentz R., Brockhaus M., Schlaeger E. J., Grau G., Piguet P. F., Pointaire P., Vassalli P., Loetscher H. Recombinant soluble tumor necrosis factor receptor proteins protect mice from lipopolysaccharide-induced lethality. Eur J Immunol. 1991 Nov;21(11):2883–2886. doi: 10.1002/eji.1830211134. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Nophar Y., Kemper O., Brakebusch C., Englemann H., Zwang R., Aderka D., Holtmann H., Wallach D. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R, cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J. 1990 Oct;9(10):3269–3278. doi: 10.1002/j.1460-2075.1990.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Matsuyama T., Mori S., Hamamoto Y., Kobayashi N., Yamamoto N., Josephs S. F., Wong-Staal F., Shimotohno K. Augmentation of human immunodeficiency virus type 1 gene expression by tumor necrosis factor alpha. AIDS Res Hum Retroviruses. 1989 Apr;5(2):131–138. doi: 10.1089/aid.1989.5.131. [DOI] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Kinter A., Justement J. S., Kehrl J. H., Bressler P., Stanley S., Fauci A. S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Yasukawa K., Suzuki H., Futatsugi K., Fukunaga T., Yokomizo C., Koishihara Y., Fukui H., Ohsugi Y., Yawata H. Preparation of soluble murine IL-6 receptor and anti-murine IL-6 receptor antibodies. J Immunol. 1991 Jul 1;147(1):168–173. [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Scott-Algara D., Vuillier F., Marasescu M., de Saint Martin J., Dighiero G. Serum levels of IL-2, IL-1 alpha, TNF-alpha, and soluble receptor of IL-2 in HIV-1-infected patients. AIDS Res Hum Retroviruses. 1991 Apr;7(4):381–386. doi: 10.1089/aid.1991.7.381. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem. 1987 May 25;262(15):6951–6954. [PubMed] [Google Scholar]
- Tadmori W., Mondal D., Tadmori I., Prakash O. Transactivation of human immunodeficiency virus type 1 long terminal repeats by cell surface tumor necrosis factor alpha. J Virol. 1991 Dec;65(12):6425–6429. doi: 10.1128/jvi.65.12.6425-6429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Sydow M., Sönnerborg A., Gaines H., Strannegård O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991 Apr;7(4):375–380. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]



