Table 1. Optimization of the reaction conditions a .
| |||||
| Entry | Catalyst (mol%) | Additives (equiv.) | Solvent | T (°C) | Yield b (%) |
| 1 | TBAI (20) | — | MeCN | 70 | 18 |
| 2 | TBAI (20) | — | DCM | 70 | 10 |
| 3 | TBAI (20) | — | 1,4-Dioxane | 70 | Trace |
| 4 | TBAI (20) | — | Toluene | 70 | 0 |
| 5 | TBAI (20) | — | MeCN | 100 | 25 |
| 6 | I2 (15) | — | MeCN | 100 | Messy |
| 7 | KI (20) | — | MeCN | 100 | Messy |
| 8 | CuI (20) | — | MeCN | 100 | 16 |
| 9 | TBAI (20) | HOAc (1.0) | MeCN | 100 | 28 |
| 10 | TBAI (20) | l-Proline (1.0) | MeCN | 100 | 33 |
| 11 | TBAI (20) | PivOH (1.0) | MeCN | 100 | 35 |
| 12 | TBAI (20)/CuI (5) | PivOH (1.0) | MeCN | 100 | 49 |
| 13 | TBAI (30)/Cu(OAc)2 (5) | PivOH (1.0) | MeCN | 100 | 53 |
| 14 | TBAI (20)/Cu(OAc)2 (5) | PivOH (1.0) | MeCN | 100 | 61 |
| 15 | TBAI (20)/Cu(OAc)2 (5) | PivOH (2.0) | MeCN | 100 | 71 |
| 16 | TBAI (20)/Cu(OAc)2 (10) | PivOH (2.0) | MeCN | 100 | 63 |
| 17 | TBAI (20)/Cu(OAc)2 (5) | — | MeCN | 100 | 33 |
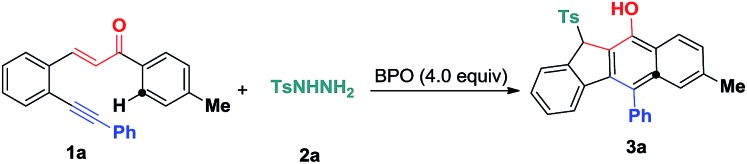
aReaction conditions: 1,5-conjugated enyne (1a, 0.25 mmol), tosylhydrazide (2a, 0.50 mmol), BPO (1.0 mmol), solvent (2.5 mL), 12 h.
bIsolated yields based on 1.
