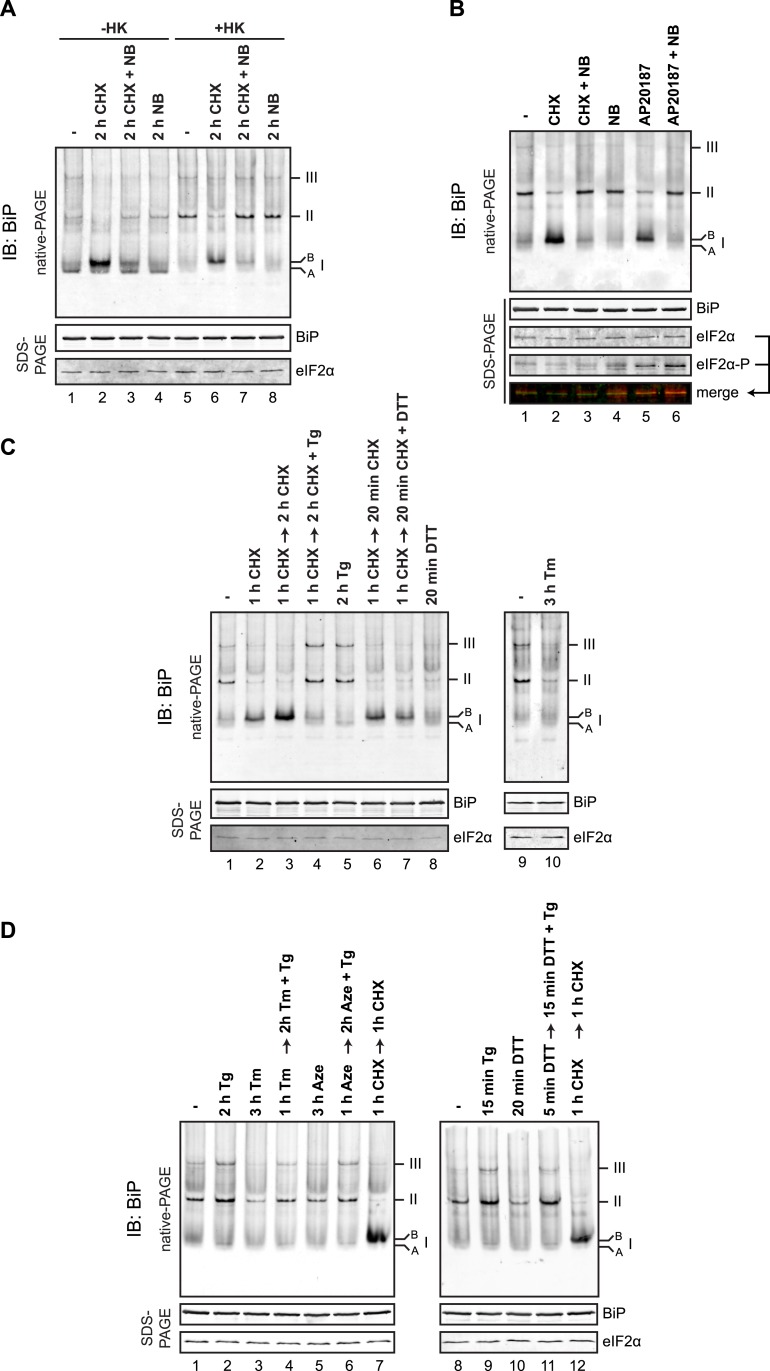
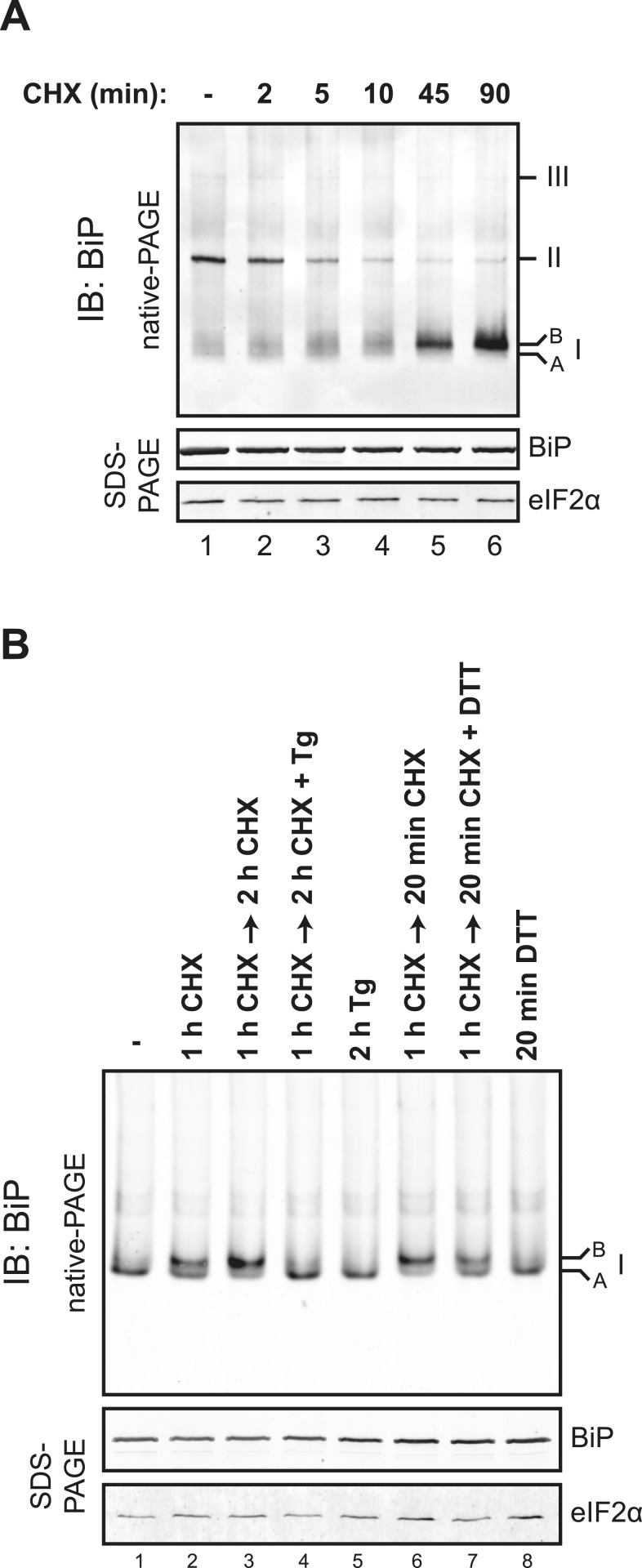
Figure 8. BiP oligomeric status responds to changes in ER unfolded protein load.
(A) Immunoblot of endogenous BiP from CHO-K1 cell lysates resolved by native-PAGE. Where indicated the cells were exposed to cycloheximide (CHX, 100 µg/ml), novobiocin (NB, 0.5 mM) or both and the lysate was depleted of ATP by incubation with hexokinase and glucose (+HK). The major species observed on the native gel are numbered by order of descending mobility (I-III) and the ‘B’ form associated with cycloheximide and ‘A’ form present in untreated cells are noted. Immunoblot of the same sample resolved by SDS-PAGE (lower panel) reports on total BiP loaded and on eIF2α as a loading control. (B) As in ‘A’. ATP-depleted lysates of CHO-K1 cells harboring a stable Fv2E-PERK transgene encoding a dimerizer drug (AP20187)-inducible form of the eIF2α-kinase PERK. Where indicated the cells were exposed for 2 hr to AP20187 (15 nM) to activate Fv2E-PERK, in the presence or absence of novobiocin. The lower panels report on the levels of BiP, total eIF2α and phosphorylated eIF2α in the lysates. (C, D) As in ‘A’. Where indicated, cells were treated alone or sequentially with cycloheximide (CHX, 100 µg/ml) and the lumenal calcium depleting agent thapsigargin (Tg, 0.5 µM), the reducing agent DTT (1 mM) that interferes with disulfide bond formation, the glycosylation inhibitor tunicamycin (Tm, 2.5 µg/ml) or the protein misfolding-inducing proline analog, azetidine (Aze, 4 mM) in the indicated order and time. ATP was depleted from all samples during cell lysis.